Synthesis and Anti-Trypanosoma cruzi Activity of Diaryldiazepines
Abstract
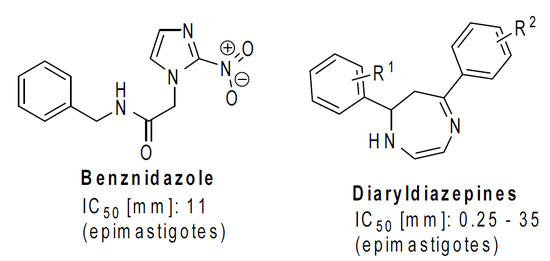
:1. Introduction



2. Results and Discussion

| Compound | Trypanocidal Activity IC50 (μM) |
|---|---|
| Benznidazole | 10.8 |
| 4a | 10.6 |
| 4b | 0.25 |
| 4c | 34.2 |
| 4d | 26.8 |
| 4e | 4.2 |
| 4f | Not Active |
3. Experimental Section
3.1. Chemistry
3.2. Pharmacology
3.3. Anti-Trypanosoma cruzi Activity
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nakanishi, M.; Tahara, T.; Araki, K.; Shiroki, M.; Tsumagari, T.; Takigawa, T. Psychotropic drugs. 18. Synthesis and structure-activity relations of 5-phenyl-1,3-dihydro-2H-thieno[2,3-e][1,4]diazepin-2-ones. J. Med. Chem. 1973, 16, 214–219. [Google Scholar]
- Eweas, A.F.; Allam, G.; Abuelsaad, A.S.; ALGhamdi, A.H.; Maghrabi, I.A. Design, synthesis, anti-schistosomal activity and molecular docking of novel 8-hydroxyquinoline-5-sufonyl 1, 4-diazepine derivatives. Bioorg. Chem. 2013, 46, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Orzaez, M.; Sancho, M.; Messeguer, A. Synthesis of enantiomerically pure perhydro-1,4-diazepine-2,5-dione and 1,4-piperazine-2,5-dione derivatives exhibiting potent activity as apoptosis inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 7097–7099. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, F.; Laleu, B.; Orchard, M.; Fioraso-Cartier, L.; Cagnon, L.; Houngninou-Molango, S.; Gradia, A.; Duboux, G.; Merlot, C.; Heitz, F.; et al. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg. Med. Chem. 2011, 19, 6989–6999. [Google Scholar]
- El-Subbagh, H.I.; Hassan, G.S.; El-Azab, A.S.; Abdel-Aziz, A.A.-M.; Kadi, A.A.; Al-Obaid, A.M.; Al-Shabanah, O.A.; Sayed-Ahmed, M.M. Synthesis and anticonvulsant activity of some new thiazolo[3,2-a][1,3]diazepine, benzo[d]thiazolo[5,2-a][12,6]diazepine and benzo[d]oxazolo[5,2-a][12,6]diazepine analogues. Eur. J. Med. Chem. 2011, 46, 5567–5572. [Google Scholar] [CrossRef] [PubMed]
- Ramajayam, R.; Giridhar, R.; Yadav, M.R.; Djaballah, H.; Shum, D.; Radu, C. Synthesis and antiproliferative activity of some diaryldiazepines and diarylpyrimidines. J. Enzym. Inhib. Med. Chem. 2007, 22, 716–721. [Google Scholar] [CrossRef]
- Chagas, C. Nova tripanossomíase humana, Estudos sobre a morfologia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolójico de nova entidade mórbida do homem. Mem. Inst. Oswaldo Cruz. 1909, 1, 159–218. [Google Scholar] [CrossRef]
- World Health Organization (2010). First WHO Report on Neglected Tropical Diseases: Working to Overcome the Global Impact of Neglected Tropical Diseases. Available online: http://whqlibdoc.who.int/publications/2010/9789241564090_eng.pdf (accessed on 14 October 2014).
- Hotez, P.J.; Dumonteil, E.; Woc-Colburn, L.; Serpa, J.A.; Bezek, S.; Edwards, M.S.; Hallmark, C.J.; Musselwhite, L.W.; Flink, B.J.; Bottazzi, M.E. Chagas Disease: “The New HIV/AIDS of the Americas”. PLoS Negl. Trop. Dis. 2012, 6, e1498. [Google Scholar] [CrossRef] [PubMed]
- Rodriques Coura, J.; de Castro, S.L. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 2002, 97, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Kelly, J.M. Trypanocidal drugs: Mechanisms, resistance and new targets. Expert Rev. Mol. Med. 2009, 11. [Google Scholar] [CrossRef]
- Moreira, D.R.M.; Lima Leite, A.C.; Cardoso, M.V.O.; Srivastava, R.M.; Hernandes, M.Z.; Rabello, M.M.; da Cruz, L.F.; Ferreira, R.S.; de Simone, C.A.; Meira, C.S.; et al. Structural Design, Synthesis and Structure–Activity Relationships of Thiazolidinones with Enhanced Anti-Trypanosoma cruzi Activity. ChemMedChem 2014, 9, 177–188. [Google Scholar]
- Franklim, T.N.; Freire-de-Lima, L.; de Nazareth Sá Diniz, J.; Previato, J.O.; Castro, R.N.; Mendonça-Previato, L.; de Lima, M.E.F. Design, Synthesis and Trypanocidal Evaluation of Novel 1,2,4-Triazoles-3-thiones Derived from Natural Piperine. Molecules 2013, 18, 6366–6382. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, A.T.; Cristian, O.S.; Karina, V.; Christian, E.-B.; Jorge, S.-D.; Javier, V.; Estefanía, B.; Hugo, C.; Mercedes, G.; Margot, P. Synthesis and biological characterization of new aryloxyindole-4,9-diones as potent trypanosomicidal agents. Bioorg. Med. Chem. Lett. 2014, 24, 3919–3922. [Google Scholar] [CrossRef] [PubMed]
- Caputto, M.E.; Ciccarelli, A.; Frank, F.; Moglionia, A.G.; Moltrasio, G.Y. Synthesis and biological evaluation of some novel 1-indanone thiazolylhydrazone derivatives as anti-Trypanosoma cruzi agents. Eur. J. Med. Chem. 2012, 55, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.M.; Moreira, D.R.M.; de Simone, C.A.; Ferreira, R.S.; McKerrow, J.H.; Meira, C.S.; Guimaraes, E.T.; Soares, M.B.P. Optimization of anti-Trypanosoma cruzi oxadiazoles leads to identification of compounds with efficacy in infected mice. Bioorg. Med. Chem. 2012, 20, 6423–6433. [Google Scholar] [CrossRef]
- Sandes, J.M.; Borges, A.R.; Junior, C.G.; Silva, F.P.; Carvalho, G.A. 3-Hydroxy-2-methylene-3-(4-nitrophenylpropanenitrile): A new highly active compound against epimastigote and trypomastigote form of Trypanosoma cruzi. Bioorg. Chem. 2010, 38, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.S. Flavone. Org. Synth. 1952, 32, 72–74. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4a–f are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes, J.C.L.; Vaz, L.B.A.; De Abreu Vieira, P.M.; Da Silva Fonseca, K.; Carneiro, C.M.; Taylor, J.G. Synthesis and Anti-Trypanosoma cruzi Activity of Diaryldiazepines. Molecules 2015, 20, 43-51. https://doi.org/10.3390/molecules20010043
Menezes JCL, Vaz LBA, De Abreu Vieira PM, Da Silva Fonseca K, Carneiro CM, Taylor JG. Synthesis and Anti-Trypanosoma cruzi Activity of Diaryldiazepines. Molecules. 2015; 20(1):43-51. https://doi.org/10.3390/molecules20010043
Chicago/Turabian StyleMenezes, Júlio César L., Luana Beatriz A. Vaz, Paula Melo De Abreu Vieira, Kátia Da Silva Fonseca, Cláudia Martins Carneiro, and Jason G. Taylor. 2015. "Synthesis and Anti-Trypanosoma cruzi Activity of Diaryldiazepines" Molecules 20, no. 1: 43-51. https://doi.org/10.3390/molecules20010043
APA StyleMenezes, J. C. L., Vaz, L. B. A., De Abreu Vieira, P. M., Da Silva Fonseca, K., Carneiro, C. M., & Taylor, J. G. (2015). Synthesis and Anti-Trypanosoma cruzi Activity of Diaryldiazepines. Molecules, 20(1), 43-51. https://doi.org/10.3390/molecules20010043