Oligomeric Procyanidins Interfere with Glycolysis of Activated T Cells. A Novel Mechanism for Inhibition of T Cell Function
Abstract
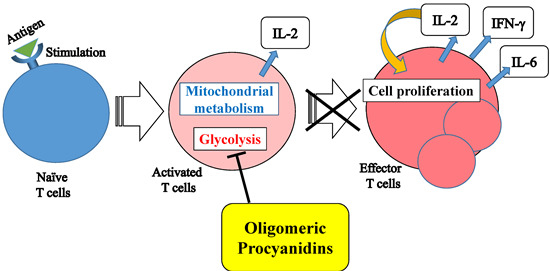
:1. Introduction
2. Results
2.1. Apple Procyanidins (APCs) Suppress Splenic T Cell Proliferation without Reducing IL-2 Secretion


2.2. Oligomeric Procyanidins Suppress T Cell Proliferation without Reducing IL-2 Secretion

2.3. Oligomeric Procyanidins Reduce Effector Cytokine Secretion

2.4. Pentameric Procyanidin Reduces the Glycolytic Activity of Activated CD4+ T Cells
3. Discussion

4. Experimental Section
4.1. Reagents
4.2. Preparation of Mice Splenocytes and Splenic CD4+ T Cells
4.3. Cell Cultures and Treatment
4.4. Measurement of Cell Proliferation
4.5. Cytokine Measurements
4.6. Measurement of l-Lactate
4.7. Measurement of Glucose Uptake
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gerriets, V.A.; Rathmell, J.C. Metabolic pathways in T cell fate and function. Trends Immunol. 2012, 33, 168–173. [Google Scholar] [PubMed]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013, 38, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Curtis, J.D.; Maggi, L.B., Jr.; Faubert, B.; Villarino, A.V.; O’Sullivan, D.; Huang, S.C.; van der Windt, G.J.; Blagih, J.; Qiu, J.; et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013, 153, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, T.; Zhu, L.; Zhao, Y. Cellular metabolism on t-cell development and function. Int. Rev. Immunol. 2014, 34, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Hammerstone, J.F.; Lazarus, S.A.; Schmitz, H.H. Procyanidin content and variation in some commonly consumed foods. J. Nutr. 2000, 130, 2086S–2092S. [Google Scholar] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [PubMed]
- Shoji, T.; Mutsuga, M.; Nakamura, T.; Kanda, T.; Akiyama, H.; Goda, Y. Isolation and structural elucidation of some procyanidins from apple by low-temperature nuclear magnetic resonance. J. Agric. Food Chem. 2003, 51, 3806–3813. [Google Scholar] [CrossRef] [PubMed]
- Lotito, S.B.; Actis-Goretta, L.; Renart, M.L.; Caligiuri, M.; Rein, D.; Schmitz, H.H.; Steinberg, F.M.; Keen, C.L.; Fraga, C.G. Influence of oligomer chain length on the antioxidant activity of procyanidins. Biochem. Biophys. Res. Commun. 2000, 276, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Sasaki, K.; Ide, K.; Matsukura, Y.; Shijima, K.; Fujiwara, D. Highly oligomeric procyanidins ameliorate experimental autoimmune encephalomyelitis via suppression of th1 immunity. J. Immunol. 2006, 176, 5797–5804. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Santamaria, A.R.; D’Auria, F.D.; Mulinacci, N.; Innocenti, M.; Cecchini, F.; Pericolini, E.; Gabrielli, E.; Panella, S.; Antonacci, D.; et al. Evaluation of anti-candida activity of vitis vinifera l. Seed extracts obtained from wine and table cultivars. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef] [PubMed]
- Guyot, S.; Marnet, N.; Sanoner, P.; Drilleau, J.F. Variability of the polyphenolic composition of cider apple (malus domestica) fruits and juices. J. Agric. Food Chem. 2003, 51, 6240–6247. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Bai, B.R.; Devaraj, S.N. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats. Int. J. Cardiol. 2007, 115, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Corder, R.; Mullen, W.; Khan, N.Q.; Marks, S.C.; Wood, E.G.; Carrier, M.J.; Crozier, A. Oenology: Red wine procyanidins and vascular health. Nature 2006, 444, 566. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.R.; Heiss, C.; Kelm, M.; Keen, C.L. The potential of flavanol and procyanidin intake to influence age-related vascular disease. J. Nutr. Gerontol. Geriatr. 2012, 31, 290–323. [Google Scholar] [CrossRef] [PubMed]
- Erlejman, A.G.; Jaggers, G.; Fraga, C.G.; Oteiza, P.I. Tnfalpha-induced NF-kappab activation and cell oxidant production are modulated by hexameric procyanidins in caco-2 cells. Arch. Biochem. Biophys. 2008, 476, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Micaelo, N.; Gonzalez-Abuin, N.; Ardevol, A.; Pinent, M.; Blay, M.T. Procyanidins and inflammation: Molecular targets and health implications. Biofactors 2012, 38, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ramljak, D.; Romanczyk, L.J.; Metheny-Barlow, L.J.; Thompson, N.; Knezevic, V.; Galperin, M.; Ramesh, A.; Dickson, R.B. Pentameric procyanidin from theobroma cacao selectively inhibits growth of human breast cancer cells. Mol. Cancer Ther. 2005, 4, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Procyanidins as antioxidants and tumor cell growth modulators. J. Agric. Food Chem. 2006, 54, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Mantena, S.K.; Baliga, M.S.; Katiyar, S.K. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis 2006, 27, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, R.; Hatano, T.; Ito, H.; Yoshida, T.; Akagi, M. Effects of tannins and related polyphenols on superoxide-induced histamine release from rat peritoneal mast cells. Phytomedicine 2000, 7, 297–302. [Google Scholar] [CrossRef]
- Nakano, N.; Nishiyama, C.; Tokura, T.; Nagasako-Akazome, Y.; Ohtake, Y.; Okumura, K.; Ogawa, H. Procyanidin c1 from apple extracts inhibits fc epsilon ri-mediated mast cell activation. Int. Arch. Allergy Immunol. 2008, 147, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kripa, K.G.; Chamundeeswari, D.; Thanka, J.; Uma Maheswara Reddy, C. Modulation of inflammatory markers by the ethanolic extract of leucas aspera in adjuvant arthritis. J. Ethnopharmacol. 2011, 134, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.P.; Loftus, R.M.; Keating, S.E.; Liou, K.T.; Biron, C.A.; Gardiner, C.M.; Finlay, D.K. Mtorc1-dependent metabolic reprogramming is a prerequisite for nk cell effector function. J. Immunol. 2014, 193, 4477–4484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Yang, L.; Dong, Y.; Zhang, Y.; Xie, Z. Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa b pathway in neuroglioma cells. Br. J. Anaesth. 2013, 110, i82–i91. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, T.; Hatakeyama, K.; Nagamachi, S.; Sekita, Y.; Sakamoto, S.; Endo, G.J.; Nishimura, M.; Matsuyama, M.; Yoshimura, K.; Sato, Y.; et al. Inhibition of development of abdominal aortic aneurysm by glycolysis restriction. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Trevillyan, J.M.; Lu, Y.L.; Atluru, D.; Phillips, C.A.; Bjorndahl, J.M. Differential inhibition of T cell receptor signal transduction and early activation events by a selective inhibitor of protein-tyrosine kinase. J. Immunol. 1990, 145, 3223–3230. [Google Scholar] [PubMed]
- Li, S.Y.; Teh, B.S.; Seow, W.K.; Liu, Y.L.; Thong, Y.H. In vitro immunopharmacological profile of the plant flavonoid baohuoside-1. Int. J. Immunopharmacol. 1991, 13, 129–134. [Google Scholar] [CrossRef]
- Huang, H.C.; Huang, Y.L.; Chang, J.H.; Chen, C.C.; Lee, Y.T. Possible mechanism of immunosuppressive effect of scoparone (6,7-dimethoxycoumarin). Eur. J. Pharmacol. 1992, 217, 143–148. [Google Scholar] [CrossRef]
- Henderson, D.J.; Naya, I.; Bundick, R.V.; Smith, G.M.; Schmidt, J.A. Comparison of the effects of FK-506, cyclosporin a and rapamycin on IL-2 production. Immunology 1991, 73, 316–321. [Google Scholar] [PubMed]
- Chaudhri, G.; Clark, I.A.; Hunt, N.H.; Cowden, W.B.; Ceredig, R. Effect of antioxidants on primary alloantigen-induced t cell activation and proliferation. J. Immunol. 1986, 137, 2646–2652. [Google Scholar] [PubMed]
- Dornand, J.; Gerber, M. Inhibition of murine t-cell responses by anti-oxidants: The targets of lipo-oxygenase pathway inhibitors. Immunology 1989, 68, 384–391. [Google Scholar] [PubMed]
- Chaudhri, G.; Hunt, N.H.; Clark, I.A.; Ceredig, R. Antioxidants inhibit proliferation and cell surface expression of receptors for interleukin-2 and transferrin in t lymphocytes stimulated with phorbol myristate acetate and ionomycin. Cell. Immunol. 1988, 115, 204–213. [Google Scholar] [CrossRef]
- Matsue, H.; Yang, C.; Matsue, K.; Edelbaum, D.; Mummert, M.; Takashima, A. Contrasting impacts of immunosuppressive agents (rapamycin, fk506, cyclosporin a, and dexamethasone) on bidirectional dendritic cell-T cell interaction during antigen presentation. J. Immunol. 2002, 169, 3555–3564. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. Hif1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and treg cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.D.; Delgoffe, G.M. The mammalian target of rapamycin: Linking T cell differentiation, function, and metabolism. Immunity 2010, 33, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Duvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mtor complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.D.; Heikamp, E.B.; Pollizzi, K.N.; Waickman, A.T. A modified model of T-cell differentiation based on mtor activity and metabolism. Cold Spring Harb. Symp. Quant. Biol. 2013, 78, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Frauwirth, K.A.; Riley, J.L.; Harris, M.H.; Parry, R.V.; Rathmell, J.C.; Plas, D.R.; Elstrom, R.L.; June, C.H.; Thompson, C.B. The cd28 signaling pathway regulates glucose metabolism. Immunity 2002, 16, 769–777. [Google Scholar] [CrossRef]
- Griffin, J.L.; Shockcor, J.P. Metabolic profiles of cancer cells. Nat. Rev. Cancer 2004, 4, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Delmastro-Greenwood, M.M.; Piganelli, J.D. Changing the energy of an immune response. Am. J. Clin. Exp. Immunol. 2013, 2, 30–54. [Google Scholar] [PubMed]
- Willinger, T.; Staron, M.; Ferguson, S.M.; de Camilli, P.; Flavell, R.A. Dynamin 2-dependent endocytosis sustains T-cell receptor signaling and drives metabolic reprogramming in T lymphocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Finlay, D.K. Regulation of glucose metabolism in t cells: New insight into the role of phosphoinositide 3-kinases. Front. Immunol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Tokura, T.; Nakano, N.; Ito, T.; Matsuda, H.; Nagasako-Akazome, Y.; Kanda, T.; Ikeda, M.; Okumura, K.; Ogawa, H.; Nishiyama, C. Inhibitory effect of polyphenol-enriched apple extracts on mast cell degranulation in vitro targeting the binding between ige and fcepsilonri. Biosci. Biotechnol. Biochem. 2005, 69, 1974–1977. [Google Scholar] [CrossRef] [PubMed]
- Nishizuka, T.; Fujita, Y.; Sato, Y.; Nakano, A.; Kakino, A.; Ohshima, S.; Kanda, T.; Yoshimoto, R.; Sawamura, T. Procyanidins are potent inhibitors of lox-1: A new player in the french paradox. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Kusumi, Y.; Mitsumata, M.; Li, X.K.; Fujino, M. Lysophosphatidylcholine upregulates lox-1, chemokine receptors, and activation-related transcription factors in human T-cell line jurkat. J. Thromb. Thrombol. 2008, 26, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Kanda, T.; Ohtake, Y. Apple (malus pumila) procyanidins fractionated according to the degree of polymerization using normal-phase chromatography and characterized by hplc-esi/ms and maldi-tof/ms. J. Chromatogr. A 2006, 1102, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the studied procyanidins are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, M.; Wakagi, M.; Shoji, T.; Takano-Ishikawa, Y. Oligomeric Procyanidins Interfere with Glycolysis of Activated T Cells. A Novel Mechanism for Inhibition of T Cell Function. Molecules 2015, 20, 19014-19026. https://doi.org/10.3390/molecules201019014
Goto M, Wakagi M, Shoji T, Takano-Ishikawa Y. Oligomeric Procyanidins Interfere with Glycolysis of Activated T Cells. A Novel Mechanism for Inhibition of T Cell Function. Molecules. 2015; 20(10):19014-19026. https://doi.org/10.3390/molecules201019014
Chicago/Turabian StyleGoto, Masao, Manabu Wakagi, Toshihiko Shoji, and Yuko Takano-Ishikawa. 2015. "Oligomeric Procyanidins Interfere with Glycolysis of Activated T Cells. A Novel Mechanism for Inhibition of T Cell Function" Molecules 20, no. 10: 19014-19026. https://doi.org/10.3390/molecules201019014
APA StyleGoto, M., Wakagi, M., Shoji, T., & Takano-Ishikawa, Y. (2015). Oligomeric Procyanidins Interfere with Glycolysis of Activated T Cells. A Novel Mechanism for Inhibition of T Cell Function. Molecules, 20(10), 19014-19026. https://doi.org/10.3390/molecules201019014