1. Introduction
The term ”resin” is often used to describe fragrant plant saps or exudates distinguished from other plant exudates such as gums, mucilages, oils, waxes, and latex. Plant resin is defined primarily as “a lipid-soluble mixture of volatile and non-volatile terpenoid, and/or phenolic secondary compounds that are (a) usually secreted in specialized structures located either internally or on the surface of the plant and (b) of potential significance in ecological interactions” [
1,
2]. Resins usually consist of a volatile fragrant fraction, usually called essential oil, and a non-volatile fraction, usually consisting of long-chain terpenoids. When fresh resins are translucent liquids but with time and the loss of the essential oil fraction, they turn into brown, yellow, or white solids that, by polymerization and oxidation, fossilize as amber [
3].
Resins have been used since ancient times as constituents of varnishes, cosmetics, adhesives, and as incense in ritual ceremonies in temples and churches. Resins from three important genera of the Burseraceae—Boswellia, Commiphora, and Bursera—have been, and still are, used in perfumery and particularly as incense. Boswellia resin is called frankincense, Commiphora resin is commonly known as myrrh and Bursera resin is often referred to as copal.
The word copal derives from
copalli, the Náhuatl (Atzec) term for incense. The Maya, in turn, used the term
pom [
4,
5] for the incense derived from
Protium,
Bursera, and
Pinus, depending on which resin-producing trees were most abundant in the areas where they lived. Later, the Spanish exported the term
copal to Europe [
1]. Nowadays, outside Mexico, the term is used for resins of the Fabaceae family and, generically, resins from Burseraceae are sometimes called
elemi [
6]. In Mexico and Guatemala copal derives mostly from
Bursera, from a species of
Protium (
Protium copal) and a pine species (
Pinus pseudostrobus) [
3].
Bursera’s distribution encompasses tropical regions from southern United States (southern Arizona, California, and Florida) to Peru. In Mexico, the genus is highly diverse and abundant along the Pacific slopes [
1].
Protium copal and
Pinus pseudostrobus are found in Mexico and Central America.
In marketplaces of Central Mexico it is possible to find a variety of copal types to satisfy many tastes (and budgets, as prices vary according to the quality):
copal blanco,
copal oro,
copal negro,
copal lágrima,
copal incienso,
copal de piedra [
3,
6]. Several authors have analyzed different commercial copals and found great differences in the chemical composition among samples of the same type of copal, suggesting that the same name might currently be applied to products produced from several different plant species [
3,
6]. Furthermore, Case
et al. [
6] stated that different types of copal are derived from different collecting procedures.
Copal blanco is the most common, being exuded directly from incisions made in the bark.
Copal oro is from resin that is exuded after removal of the bark.
Copal negro is beaten from bark [
3] and
copal de piedra is exuded as a defensive reaction to the attack of insects such as the Cerambycid beetle,
Chyptodes dejeani [
7].
Copal lágrima (copal in tears) is the product remaining in the recipient of collection and the incision in the bark [
8]. Interestingly, Stacey
et al. [
6] found that a commercial sample of
copal lágrima from the market of Tepoztlán, Morelos in central Mexico contained boswellic acids, commonly found in
Boswellia (another Burseraceae genus not found in Mexico).
Bursera Jacq. ex L. (family Burseraceae, order Sapindales), a monophyletic genus [
9] that consists of about 105 species, is a dominant taxon in seasonally dry tropical forests and also abundant in the deserts and oak savannahs of southern Mexico (
ca. 85 species). It has been divided into two subgenera,
B. subg.
Bursera and
B. subg.
Elaphrium. Species of subgenus
Bursera have 3–5-merous flowers, bivalvate fruits, and bark that typically exfoliates in colorful papery sheets or flakes, a trait that is responsible for their Aztec-derived name “cuajiote”, meaning
leprous tree. Subgenus
Elaphrium is characterized by a non-peeling grey-reddish rough bark, tetramerous flowers, and trivalvate fruits [
10].
Regional variation in which
Bursera species occur, as well as confusion in the literature about the species involved, has resulted in misunderstandings about which species are utilized as copal. Current efforts to disentangle this confusion include the use of chemical analytical methods to match compounds found in copal with the species that are the sources of the resin. While this is a promising method for recently collected copal, older copal samples will continue to be challenging to link to a species source. As collected resins harden, they gradually lose the volatile components that provide the main chemical distinctiveness to
Bursera species, leaving the non-volatile elements that are more species-invariant [
6]. This was explicit in results of De la Cruz-Cañizares
et al. [
11] who examined a “fresh” sample of Mexican copal (
Bursera cuneata) from a supplier of artists’ materials (Casa Sierra, Mexico DF, Mexico) and a five-year old sample from a Sonora market (Sonora, Mexico;
Table 1). They found considerable differences between the fresh and the five-year-old samples.
The aim of this paper is to review the chemical composition of resins from
Bursera species used and marketed in Mexico as
copal. Analytical data were collected from peer-reviewed papers found using SciFinder, Scopus and PubMed databases. Synonyms for
Bursera species are those reported by Espinosa [
12]. Other synonyms have been found on The Plant List web site [
13]. Unless otherwise specified, common names are those reported in the “Excel” file found on the CONABIO web page [
14].
Table 1.
Comparison of a fresh sample of Bursera cuneata copal (C), a five-year-old sample (D), and samples given two different artificial treatments (sample C.1, prepared by dissolving resin in turpentine and sample C.2, obtained heating the resin at 100 °C for 10 min).
Table 1.
Comparison of a fresh sample of Bursera cuneata copal (C), a five-year-old sample (D), and samples given two different artificial treatments (sample C.1, prepared by dissolving resin in turpentine and sample C.2, obtained heating the resin at 100 °C for 10 min).
| Compound a | C | C.1 | C.2 | D |
|---|
| Verbenene | v b | | | |
| o-Cymene | v | v | v | v |
| α-Pinene | v | | | v |
| Camphene | v | | | v |
| β-Pinene | v | | | v |
| α-Phellandrene | v | | | v |
| α-Terpinene | v | | | v |
| Limonene | v | v | v | v |
| γ-Terpinene | v | | v | v |
| α-Terpinolene | v | v | v | v |
| Verbenone | v | | | |
| Carvacrol | v | | | |
| Sabinol | v | | | |
| 4-Terpineol | v | | | v |
| Carvacrol methyl ether | v | | | |
| Fenchyl acetate | v | | | |
| cis-Calamenene | v | | | |
| Isoledene | v | | | |
| trans-Caryophyllene | | | | v |
| Hexanedioic acid, bis(2-ethylhexyl) ester | | | | v |
2. Copal Species, Distribution and Composition
Linares and Bye [
15] described extensively how “copaleros” collect the resin. They use a particular knife (quixala or quichala) to make incisions into the bark. They put a leaf of
Quercus glaucoides under the cut to isolate the resin from impurities on the bark. To collect the liquid resin, they employ leaves of
Agave angustifolia. They collect copal from July to September every year. To avoid killing or damaging the trees, resin is only collected from the same tree every two or three years [
15]. These authors reported that the most appreciated species are
B. bipinnata (Sessè & Moc. ex DC.) Engl. and
B. copallifera (Sessè & Moc. ex DC.) Bullock. Nowadays, painters use
copal as a binding medium for paint together with linseed oil.
Most of the phytochemical studies to identify the species used as
copal have been done on commercial samples and on archeological Aztec objects [
6,
16]. “Fresh” resins have a characteristic pine-lemony smell due to volatile terpenes and alkanes such as α-pinene, β-phellandrene, limonene, δ-carene, and heptane [
17], while “aged” resins are studied for the triterpenoidic composition of the non-volatile fraction [
11].
Pinus resins, are characterized by a large volatile fraction (20%–50%) with monoterpenes predominating over sesquiterpenes while in the non-volatile component diterpene acids with abietane, pimarane, and labdane frameworks are common. Burseraceae resins contain mono- and sesquiterpenes in the volatile fraction and triterpenoids in the non-volatile fraction [
1]. Particularly,
Protium spp. terpenoids are dominated by α- and β-amyrin and
Bursera spp. terpenoids contain lupane compounds (e.g., lupeol) [
16]. Often the non-volatile fraction of
Bursera spp. contains lignans.
2.1. Distribution, Synonyms, Common Names, and Primary Essential Oils of Described Bursera Species
A diversity of Bursera species are known to be used as incense, but only a small number are reported by several authors as commercial copal, specifically, B. linanoe, B. copallifera, B. bipinnata, and B. fagaroides. Other important incense sources that are not as commercialized are B. microphylla, B. penicillata, B. simaruba, B. schelechtendalii, and B. excelsa.
Bursera bipinnata (Sessè & Moc. ex DC.) Engl. (synonym:
Amyris bipinnata [
18], subg.
Elaphrium) is commonly known as
copal cimarrón and
copal santo. It is distributed from southern Chihuahua and Sinaloa to Morelos, Guerrero, Oaxaca, and Chiapas. Although chemically variable, the main volatile component of its fresh resin is α-pinene (Becerra, J.X., Personal observations. 2015).
Bursera copallifera (Sessè & Moc. ex DC.) Bullock (synonyms:
B. jorullensis,
Bullockia jorullensis,
B. palmeri var.
glabrescens,
Elaphrium copalliferum and
E. jorullense [
14], subg.
Elaphrium) is commonly known as
copal ancho but also
c'uájtsutacu (Tarasco name) and
copalcuáuitl-patlahoac (Náhuatl name) [
12,
19]. It is native to the dry forests from the Mexican states from Nayarit to Oaxaca and Puebla at altitudes of between 1000 and 1900 m. Its essential oil is rich in germacrene D and α-humulene [
20].
Bursera cuneata (Schltdl.) Engl. (synonyms:
Elaphrium cuneatum [
14], subg.
Elaphrium) is commonly known as
copal,
copalillo,
cuerecatzundi,
cuerica-tzunda,
cuiricatzunda (Purépecha name) [
18]. It is native to the Mexican oak-tropical deciduous forest transition zone from Jalisco to Oaxaca. Its essential oil is relatively abundant in α-pinene, β-caryophyllene, and germacrene D (Becerra, J.X., Personal observations. 2015).
Bursera excelsa (Kunth) Engl. (synonyms:
Bullockia sphaerocarpa and
Elaphrium excelsum [
14], subg.
Elaphrium) is commonly known as
tecomahaca and
copalquín in Náhuatl language. It is largely present along the pacific coast of Mexico (Nayarit, Chiapas, Jalisco, Durango,
etc.). Its fresh resin is rich in germacrene D and β-caryophyllene [
20].
Bursera fagaroides (H.B.K.) Engl., or “fragrant bursera” (synonyms
B. obovata, B. schaffneri [
21], subg.
Bursera) exists in three different varieties:
elongata,
fagaroides, and
purpusii [
22]. CONABIO and The Plant List database report several synonyms, such as
B. lonchophylla, B. tenuifolia,
B. schafferii,
Elaphrium covillei,
E. inaguense,
Amyris ventricosa [
13,
14]. It is commonly known as
aceitillo,
copa,
cuajiote amarillo and
jiote (Náhuatl name) [
14]. It is native to northern Mexico, (Sinaloa, Sonora) and the central and southern states (Queretaro, Guerrero, Jalisco, Michoacan, Nayarit, Oaxaca,
etc.). The volatile chemistry of this taxon is highly variable, but plants often contain large amounts of α-pinene, β-phelandrene, germacrene B, and germacrene D (Becerra, J.X., Personal observations. 2015). Copal studies on
B. fagaroides most often do not identify variety investigated.
Bursera linanoe (La Llave) Rzed., Calderón & Medina (synonyms:
B. aloexylon,
B. delpechiana,
B. longipedunculata,
Amyris linaloe,
Elaphrium longipedunculatum [
13], subg.
Elaphrium), also known as Indian lavender tree. This is one of the species most extensively used as copal by the indigenous Mexican people in the past as well as in the present. The XVI century Spanish historian Francisco Hernandez describes this species known to the Aztecs as “
Copalcuáuitl”, meaning
copalli tree, now commonly known as
copal blanco [
19]. Their drawings of the plant source of this copal also closely resemble live
B. linanoe trees, confirming its identity. This species produces one of the most pleasant and fragrant resins and is currently cultivated in India for use in the perfume industry [
23]. It is the only
Bursera species whose essential oil consists predominantly of linalyl acetate [
23,
24].
Bursera microphylla A. Gray (synonyms:
Elaphrium microphyllum,
Terebinthus microphylla, subg.
Bursera) is commonly known as elephant tree,
torote,
torote blanco,
copal, or
cuajiote colorado and is native to the Sonoran Desert, from southwestern Arizona and southeastern California, to the western Mexican mainland, and Baja California [
16,
25,
26]. The chemistry of this species also varies greatly among geographic locations. The resin acetonic extracts of different samples from two different populations (Guaymas and La Paz) were studied by Mooney and Emboden [
27] who identified α-pinene, β-pinene, phellandrene, limonene, cineole, and four unidentified compounds, while Tucker and coll. [
28] found that plant samples from Southern Arizona were rich in β-caryophyllene.
Bursera penicillata (Sessè & Moc. ex DC.) Engl. (synonyms:
Amyris penicillata,
Bursera mexicana,
Elaphrium delpechianum,
E. mexicanum,
E. penicillatum,
Terebinthus delpechiana,
T. mexicana [
13,
14], subg.
Elaphrium) is also known as
Bullockia inopinata [
14]. Its common name is
torote incienso and
torote copal. It is native to the westerns states of Sonora, Aguascalientes, Sinaloa, Nayarit, Zacatecas, Michoacan,
etc. [
21].
Bursera schlechtendalii Engl. (synonyms:
B. jonesii,
E. jonesii,
Terebinthus jonesii,
T. schlechtendalii, subg.
Bursera). It is native to Central Mexico and Guatemala. Its fresh resin contains large amounts of the highly volatile heptane, β-phellandrene, sabinene, nonane, and myrcene [
29].
Bursera simaruba (L.) Sarg. (synonyms:
B. bonairensis,
B. gummifera,
B. integerrima,
B. subpubescens,
Elaphrium arboretum,
E. integerrimum,
E. simaruba,
Terebinthus simaruba,
T. arborea [
13], subg.
Bursera) is commonly known as
chacaj or
chakaj (Tojolabal name),
yaga-guito (Zapotec name) [
14]. It has a wide distribution in Mexico and Central America. Its volatile chemistry varies among locations, but it contains α-pinene, β-pinene, and a diversity of sesquiterpenoids including α-copaene, δ-elemene, β-caryophyllene, germacrene D, germacrene B, and β-sesquiphellandrene (Becerra, J.X., Personal observations. 2015).
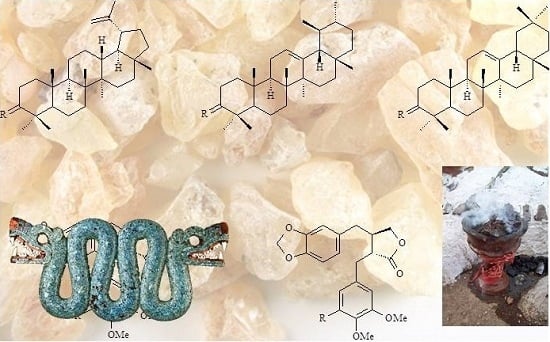
2.2. Composition of the Triterpenoid Fraction
Triterpenoid of the lupane type are characteristic of
Bursera resins, but often ursane and oleanane triterpenoids are also present (
Figure 1). Stacey
et al. [
6] examined and compared
copal resins from different ancient artefacts from the British Museum, botanical specimens from
Pinus,
Protium and
Bursera spp. and commercial samples of
copal lágrima,
copal negro,
copal incienso,
copal de piedra and
copal blanco, from the market of Tepoztlan, Morelos. They found that the samples of commercial
copal blanco,
negro and
de piedra have similar terpenoid profiles characterized by 3-
epi-β-amyrin, 3-
epi-α-amyrin, lupeol and α-amyrin, similar to that of a fifty year old
B. excelsa sample but with some affinity with
B. linanoe. The fresh sample of
B. fagaroides var.
fagaroides that they examined showed a completely different profile, dominated by oleanonic and ursonic acids. Furthermore, as mentioned above, they found that the commercial sample of
copal lágrima has a terpenoid composition resembling
Boswellia, due to the presence of boswellic acids.
Figure 1.
Triterpenoid markers used in GC-MS analysis of copal [
16].
Figure 1.
Triterpenoid markers used in GC-MS analysis of copal [
16].
Lucero-Gómez
et al. [
30] studied the triterpenoid composition of fresh samples of different
Bursera copals using GC, and comparing them to nine triterpene standards (3-
epi-β-amyrin, 3-
epi-α-amyrin, 3-
epi-lupeol, β-amyrone, β-amyrin, α-amyrone, α-amyrin, lupenone, lupeol) derivatized as trimethylsilyl ethers (OTMS).
They analyzed
B. bipinnata,
B. excelsa,
B. copallifera and
B. penicillata, but also
B. stenophylla as its botanical distinction from
B. bipinnata is unclear [
21],
B. simaruba because it was used as binder in Bonampak murals (Maya) [
16] and
B. grandifolia because it is phylogenetically related to
B. simaruba. In
Table 2 we report the phytochemical results for these species. The authors found that the GC-MS profiles of
B. bipinnata and
B. stenophylla are identical.
Table 2.
Non-volatile terpene fraction composition of
Bursera resins studied by Lucero-Gómez [
16].
Table 2.
Non-volatile terpene fraction composition of Bursera resins studied by Lucero-Gómez [16].
| Compound | B. bipinnata and B. stenophylla | B. copallifera | B. excelsa | B. penicillata | B. grandifolia | B. simaruba |
|---|
| 3-epi-β-amyrin | v a | = b | v | v | v | v |
| 3-epi-α-amyrin | v | = | v | v | v | v |
| 3-epi-lupeol | v | = | v | v | v | v |
| β-amyrone | v | = | = | v | = | v |
| β-amyrin | v | = | = | v | v | v |
| α-amyrone | v | = | = | = | = | v |
| α-amyrin | v | = | = | v | v | v |
| lupenone | v | v | = | = | = | = |
| lupeol | v | v | = | v | = | v |
They identified in
B. bipinnata all the nine standards and four more unidentified compounds (
Table 2). Studying the triterpenic profile of
B. copallifera, Lucero-Gómez
et al. found lupeol and lupenone and five other molecules. From the fragmentation pattern in GC-MS analysis, they established the nature of four of them: three urs-12-ene derivatives and one olean-12-ene derivative [
16]. In
B. excelsa they identified only three of the nine standards: 3-
epi-α- and 3-
epi-β-amyrins and 3-
epi-lupeol. Furthermore, they noted the presence of five other unidentified triterpenoids, three of which had MS fragmentation patterns similar to olean-12-ene type molecules and two of which were amyrones. In
B. penicillata, they found a greater variety of triterpenoids than other resins. The only missing standards were α-amyrone and lupenone. Furthermore, they found seven other triterpenoids, three of which were identified as olean-12-ene derivatives (
Table 2). In
B. simaruba all of the standards were found except lupenone. Five other triterpenoids were detected in this resin, one of which was identified as an urs-12-ene compound.
B. simaruba and
B. grandifolia GC profiles are different, as is shown in
Table 2.
The phytochemical composition of the non-volatile fraction of B. microphylla fresh resin collected near Hermosillo, Sonora was recently studied by our research group. The methanol extract of the resin was divided by means of solvent with different polarity into two sub-fractions: the hexane sub-fraction (H) and the dichloromethane one (DCM). The H-sub-fraction contained several terpenoids and lignans.
The presence of β-caryophyllene and caryophyllene oxide was confirmed. Known triterpenoids with a lupane (betulonic acid), oleanane (oleanonic acid), and dammarane (16,20-dihydroxy-dammarenone and mansumbinone) skeleton were isolated (
Figure 2). Furthermore, two new triterpenoids with the quite rare malabaricane skeleton (malabaricatrienone and malabaricatrienol) were present (
Figure 2). In the hexane subfraction there were several diterpenoids such as verticillene, 5-
epi-ent-verticillol (verticillane skeleton) and microphyllanin (
Figure 3) [
31]. Microphyllanin is a cembrane diterpenoid, that has been previously isolated from
B. multijuga [
32]. Peraza-Sánchez
et al. isolated in 1995 a new lupane-type triterpene from the chloroformic resin extract of
B. simaruba (
Figure 4) [
33].
Figure 2.
Triterpenoidic compounds isolated from
B. microphylla [
31].
Figure 2.
Triterpenoidic compounds isolated from
B. microphylla [
31].
Figure 3.
Diterpene compounds isolated from
B. microphylla [
31].
Figure 3.
Diterpene compounds isolated from
B. microphylla [
31].
Figure 4.
New lupane-type triterpene isolated from
B. simaruba [
33].
Figure 4.
New lupane-type triterpene isolated from
B. simaruba [
33].
2.3. Composition of the Lignan Fraction
Lignans are phenolic components of foods and medicines that arise from radical coupling of two units of coniferyl alcohol. Lignans can be classified into different groups based on skeleton oxidation and functionalization [
34,
35].
B. simaruba,
B. fagaroides and
B. microphylla exudates have been studied for lignan content. Most of the studies report the lignan content of bark, stem or leaves extracts [
36]. Velazquez-Jimenez
et al. [
37] isolated from
B. fagaroides resin two aryltetraline lignans ((−)-deoxy-podophyllotoxin, (−)-morelensin) and two dibenzylbutirolactone lignans ((−)-yatein and (−)-5′-des-methoxyyatein). The authors determined the absolute configuration of these compounds by comparison of the vibrational circular dichroism spectra of known podophyllotoxin and desoxypodophyllotoxin with those obtained by density functional theory calculations. Other diarylbutane lignans were isolated by Morales-Serna
et al. [
38] from the chloroform extract of
B. fagaroides resin: 9-acetyl-9′-pentadecanoyl-dihydroclusin, 2,3-demethoxy-secoisolintetralin monoacetate, dihydroclusin monoacetate, together with previously known 2,3-demethoxysecoisolintetralin diacetate and dihydroclusin diacetate (
Figure 5).
Figure 5.
Lignans isolated from
B. fagaroides resin [
37,
38].
Figure 5.
Lignans isolated from
B. fagaroides resin [
37,
38].
Morelensin was isolated for the first time by Jolad
et al. from dried exudate of
Bursera morelensis [
39]. Peraza-Sánchez
et al. isolated for the first time picropolygamain from the chloroformic extract of
B. simaruba resin (
Figure 6) [
40].
Figure 6.
Picropolygamain isolated from
B. simaruba [
40].
Figure 6.
Picropolygamain isolated from
B. simaruba [
40].
The phytochemical analysis of
B. microphylla hexane subfraction of a methanolic extract, led to the isolation of four known lignans: burseranin, burseran, ariensin and dihydroclusin diacetate [
31] (
Figure 7).
Figure 7.
Lignans isolated from
B. microphylla resin [
31].
Figure 7.
Lignans isolated from
B. microphylla resin [
31].
It is interesting to note that lignans isolated from copal, with the exception of burseran, belong to the aryltetraline and dibenzylbutane groups. Koulman studied the biosynthesis of
Bursera lignans from matairesinol and he classified them in four different groups (
Figure 8) [
36]. From his studies on dry leaves, Koulman noted that groups 1 and 2 lignans are present in subg.
Bursera (
B. fagaroides and
B. microphylla) while groups 3 and 4 are in subg.
Elaphrium (
B. bipinnata,
B. copallifera,
B. cuneata,
B. excelsa and
B. penicillata). The few studies on lignans isolated from
Bursera resins, are in agreement with these results. Lactone lignans isolated from
B. fagaroides and
B. microphylla (both in subg
Bursera) belong to group 1.
Figure 8.
Groups of lignans present in
Bursera spp. leaves according to Koulman [
36].
Figure 8.
Groups of lignans present in
Bursera spp. leaves according to Koulman [
36].
2.4. Biological Activities of Copal.
Case and Orta-Amaro [
3,
41] described many of the ancient and traditional uses of copal. Mesoamerican people used copal for different purposes. First of all, it was considered to be food for the gods, but it was also used as incense used during ceremonies, as a binder mixed with pigments for painting, for the decoration of murals and for the preparation of holy artifact. “Copal served as the “flesh” of idols with a wooden skeleton that were further covered with a rubber skin” [
3]. Copal smoke was used to cure headache and to clean the body after being exposed to sick people [
19]. Copal ground and dissolved in water was used by the Nahua people to treat diarrhea, as an anti-inflammatory poultice [
41], to plug tooth cavities (Nahua and Maya), and to treat pneumonia.
Bursera copallifera was used against uterine diseases.
Bursera bipinnata has been used to treat wounds and
B. simaruba has been used to treat fever and chicken pox [
3]. The Seri Pharmacopoeia reports the use of
B. microphylla resin for sore throats, headache, and for wound healing [
42]. In Oaxaca city, copal of
B. fagaroides is used to prepare infusions to treat stomach problems and as an anti-inflammatory [
38].
Copal was, and still is, primarily used as folk medicine and only a few scientific papers are found in the literature that have investigated its biological activity.
Bursera bipinnata copal was studied for its film-forming potential and its potential use as coating material for sustained release and colon-targeted drug delivery [
43]. Velazquez-Jimenez
et al. prepared an ethanol extract of the dry exudate of
B. fagaroides and found it cytotoxic, in a concentration-dependent manner, against HT-29 (human colorectal adenocarcinoma) cells with IC
50 values of 0.40 and 0.41 μg/mL after 48 and 72 h, respectively [
37]. As already mentioned, these authors isolated from this extract four podophyllotoxin related lignans from this extract [
37]. The methanol extract of
B. microphylla was studied for its cytotoxic activity against human cancer cell lines: A549 (lung cancer) (IC
50 53.77 μg/mL) andHeLa (cervix cancer) (IC
50 13.85 μg/mL), and against the murine cell lines M12.C3.F6 (B cell lymphoma) (IC
50 26.00 μg/mL). Following these encouraging preliminary results, the new and the already known compounds isolated by hexane fraction were tested for their cytotoxic activity against three human cancer cell lines, namely, A549, HeLa, and PC-3 (prostate cancer), and against the murine cell lines M12.C3.F6 and RAW264.7 (macrophages transformed by the virus Abelson leukemia). Malabricatrienone, malabaricatrienol and microphyllanin (
Figure 2 and
Figure 3) were found to be inactive, and among the known compounds, only dihydroclusin diacetate was shown to be active against murine cell line M12.C3.F3 (IC
50 2.5 μM), while ariensin, burseran, and dihydroclusin diacetate (
Figure 5) were active against the RAW246.7 murine cell line (IC
50 9.8, 0.4, and 0.2 μM, respectively). Betulonic acid (
Figure 2) was shown to be active against all the tested lines (IC
50: M12.C3.F3 = 13.2 μM, A549 = 12.6 mM, HeLa = 13.6 μM, RAW 264.7 = 10.2 μM, PC-3 = 18.6 μM) [
31].
Although few studies have been reported on the biological activities of
Bursera copal, several of the isolated compounds have been studied. Many terpenoids and lignans isolated from
Bursera copal have been studied and several reviews on their biological activity have been published. For example biological properties of lupeol, α- and β-amyrins and lignans have been recently reviewed [
44,
45,
46].