Mechanism of Breast Cancer Preventive Action of Pomegranate: Disruption of Estrogen Receptor and Wnt/β-Catenin Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. PE Suppresses Elevated ER-α and ER-β Expressions during DMBA-Induced Mammary Tumorigenesis



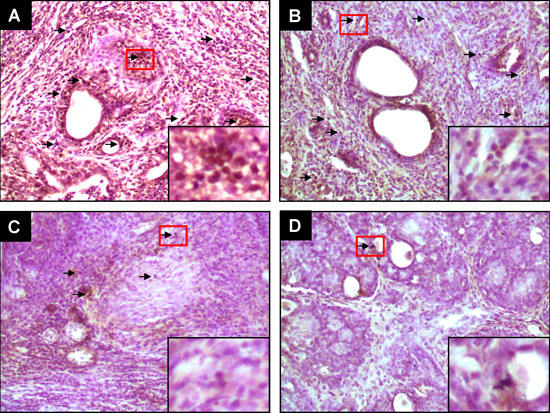
2.2. PE Interferes with Activated β-Catenin Signaling during Mammary Tumorigenesis Induced by DMBA

2.3. PE Downregulates Cyclin D1 Expression during DMBA-Induced Mammary Carcinogenesis

3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Experimental Design
4.3. Immunohistochemical Analysis
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Ottini, L. Male breast cancer: A rare disease that might uncover underlying pathways of breast cancer. Nat. Rev. Cancer 2014, 14, 643. [Google Scholar] [CrossRef] [PubMed]
- Lillie, S.E.; Brewer, N.T.; O’Neill, S.C.; Morrill, E.F.; Dees, E.C.; Carey, L.A.; Rimer, B.K. Retention and use of breast cancer recurrence risk information from genomic tests: The role of health literacy. Cancer Epidemiol. Biomark. Prev. 2007, 16, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Campeau, P.M.; Foulkes, W.D.; Tischkowitz, M.D. Hereditary breast cancer: New genetic developments, new therapeutic avenues. Hum. Genet. 2008, 124, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Bozovic-Spasojevic, I.; Azambuja, E.; McCaskill-Stevens, W.; Dinh, P.; Cardoso, F. Chemoprevention for breast cancer. Cancer Treat. Rev. 2012, 38, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Files, J.A.; Stan, D.L.; Allen, S.V.; Pruthi, S. Chemoprevention of breast cancer. Women’s Health 2012, 8, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, C.P.; Lindgren, M.E.; Shapiro, C.L.; Kiecolt-Glaser, J.K. Child maltreatment and breast cancer survivors: Social support makes a difference for quality of life, fatigue and cancer stress. Eur. J. Cancer 2012, 48, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A. Hormone replacement therapy and the risk of breast cancer. Nat. Rev. Clin. Oncol. 2011, 8, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kolonel, L.N.; Lim, U.; White, K.K.; Henderson, B.E.; Wilkens, L.R. Alcohol consumption and breast cancer risk among women from five ethnic groups with light to moderate intakes: The Multiethnic Cohort Study. Int. J. Cancer 2014, 134, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Díaz-Lagares, A.; Carreira, M.C.; Amil, M.; Casanueva, F.F. Oxidative stress associated to dysfunctional adipose tissue: A potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic. Res. 2013, 47, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Pierobon, M.; Frankenfeld, C.L. Obesity as a risk factor for triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 137, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Lu, Y.; Wu, K.; Lin, Q.; Shen, W.; Zhu, M.; Huang, S.; Chen, J. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 2013, 37, 197–206. [Google Scholar] [PubMed]
- Cazzaniga, M.; Bonanni, B. Breast cancer chemoprevention: Old and new approaches. J. Biomed. Biotechnol. 2012, 2012, 985620. [Google Scholar] [CrossRef] [PubMed]
- Eccles, S.A.; Aboagye, E.O.; Ali, S.; Anderson, A.S.; Armes, J.; Berditchevski, F.; Blaydes, J.P.; Brennan, K.; Brown, N.J.; Bryant, H.E.; et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013, 15, R92. [Google Scholar] [CrossRef] [PubMed]
- Song, J.K.; Bae, J.M. Citrus fruit intake and breast cancer risk: A quantitative systemic review. J. Breast Cancer 2013, 16, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.A.; Xue, F.; Michels, K.B. Green tea consumption and breast cancer risk or recurrence: A meta-analysis. Breast Cancer Res. Treat. 2010, 119, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Mourouti, N.; Panagiotakos, D.B. Soy food consumption and breast cancer. Maturitas 2013, 76, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Norat, T. Dietary compared with blood concentrations of carotenoids and breast cancer risk: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2012, 96, 356–373. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Flatt, S.W.; Rock, C.L.; Ritenbaugh, C.; Newman, V.; Pierce, J.P. Increased fruit, vegetable and fiber intake and lower fat intake reported among women previously treated for invasive breast cancer. J. Am. Diet Assoc. 2002, 102, 801–808. [Google Scholar] [CrossRef]
- Rock, C.L.; Demark-Wahnefried, W. Nutrition and survival after the diagnosis of breast cancer: A review of the evidence. J. Clin. Oncol. 2002, 20, 3302–3316. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.I.; Zhao, J.; Khan, I.A.; Walker, L.A.; Dasmahapatra, A.K. Potential utility of natural products as regulators of breast cancer-associated aromatase promoters. Reprod. Biol. Endocrinol. 2011, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Reuben, S.C.; Gopalan, A.; Petit, D.M.; Bishayee, A. Modulation of angiogenesis by dietary phytoconstituents in the prevention and intervention of breast cancer. Mol. Nutr. Food Res. 2012, 56, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Biswas, J.; Sung, B.; Aggarwal, B.B.; Bishayee, A. Chemopreventive and chemotherapeutic potential of curcumin in breast cancer. Curr. Drug Targets 2012, 13, 1799–1819. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, H.S.; Warri, A.M.; Woode, D.R.; Hilakivi-Clarke, L.; Clarke, R. Influence of berry polyphenols on receptor signaling and cell-death pathways: Implications for breast cancer prevention. J. Agric. Food Chem. 2012, 60, 5693–5708. [Google Scholar] [CrossRef] [PubMed]
- Vadodkar, A.S.; Suman, S.; Lakshmanaswamy, R.; Damodaran, C. Chemoprevention of breast cancer by dietary compounds. Anticancer Agents Med. Chem. 2012, 12, 1185–1202. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Sakakibara, H.; Yamazaki, S.; Shimoi, K. Breast cancer and flavonoids—A role in prevention. Curr. Pharm. Des. 2013, 19, 6125–6132. [Google Scholar] [CrossRef] [PubMed]
- Yiannakopoulou, E.C. Effect of green tea catechins on breast carcinogenesis: A systematic review of in vitro and in vivo experimental studies. Eur. J. Cancer Prev. 2014, 23, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.R.; Mandal, A.; Bhatia, D.; Siveen, K.S.; Sethi, G.; Bishayee, A. Oleanane triterpenoids in the prevention and therapy of breast cancer: Current evidence and future perspectives. Phytochem. Rev. 2014, 13, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Kado, K.; Forsyth, A.; Patel, P.R.; Schwartz, J.A. Dietary supplements and natural products in breast cancer trials. Front. Biosci. 2012, 4, 546–567. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Singh, A.; Chagtoo, M.; Singh, N.; Godbole, M.M.; Chakravarti, B. Phytochemicals for breast cancer therapy: Current status and future implications. Curr. Cancer Drug Targets 2015, 15, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar] [PubMed]
- Johanningsmeier, S.D.; Harris, G.K. Pomegranate as a functional food and nutraceutical source. Annu. Rev. Food Sci. Technol. 2011, 2, 181–201. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Calhau, C. The bioactivity of pomegranate: Impact on health and disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Chamcheu, J.C.; Adhami, V.M.; Mukhtar, H. Pomegranate extracts and cancer prevention: molecular and cellular activities. Anticancer Agents Med. Chem. 2013, 13, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential effects of pomegranate polyphenols in cancer prevention and therapy. Oxid. Med Cell. Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.D.; Mehta, R.; Yu, W.; Neeman, I.; Livney, T.; Amichay, A.; Poirier, D.; Nicholls, P.; Kirby, A.; Jiang, W.; et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res. Treat. 2002, 71, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Toi, M.; Bando, H.; Ramachandran, C.; Melnick, S.J.; Imai, A.; Fife, R.S.; Carr, R.E.; Oikawa, T.; Lansky, E.P. Preliminary studies on the anti-angiogenic potential of pomegranate fractions in vitro and in vivo. Angiogenesis 2003, 6, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.N.; Gorin, M.A.; Rosenthal, D.; Pan, Q.; Bao, L.W.; Wu, Z.F.; Newman, R.A.; Pawlus, A.D.; Yang, P.; Lansky, E.P.; et al. Pomegranate fruit extract impairs invasion and motility in human breast cancer. Integr. Cancer Ther. 2009, 8, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Zhang, Y.; Seeram, N.P.; Heber, D.; Chen, S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev. Res. 2010, 3, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Nair, V.; Khan, M.; Ciolino, H.P. Pomegranate extract inhibits the proliferation and viability of MMTV-Wnt-1 mouse mammary cancer stem cells in vitro. Oncol. Rep. 2010, 24, 1087–1091. [Google Scholar] [PubMed]
- Dikmen, M.; Ozturk, N.; Ozturk, Y. The antioxidant potency of Punica granatum L. fruit peel reduces cell proliferation and induces apoptosis on breast cancer. J. Med. Food 2011, 14, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.M.; Aravind, S.R.; Varghese, S.; Mini, S.; Sreelekha, T.T. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 2012, 5, 489–496. [Google Scholar] [PubMed]
- Banerjee, N.; Talcott, S.; Safe, S.; Mertens-Talcott, S.U. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: Potential role of miRNA-27a and miRNA-155 in cell survival and inflammation. Breast Cancer Res. Treat. 2012, 136, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Wang, L.; Penichet, M.; Martins-Green, M. Pomegranate juice and specific components inhibit cell and molecular processes critical for metastasis of breast cancer. Breast Cancer Res. Treat. 2012, 136, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Sreeja, S.; Santhosh Kumar, T.R.; Lakshmi, B.S.; Sreeja, S. Pomegranate extract demonstrate a selective estrogen receptor modulator profile in human tumor cell lines and in vivo models of estrogen deprivation. J. Nutr. Biochem. 2012, 23, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Shirode, A.B.; Kovvuru, P.; Chittur, S.V.; Henning, S.M.; Heber, D.; Reliene, R. Antiproliferative effects of pomegranate extract in MCF-7 breast cancer cells are associated with reduced DNA repair gene expression and induction of double strand breaks. Mol. Carcinog. 2014, 53, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Lansk, E.P. Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. Eur. J. Cancer Prev. 2004, 13, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Mandal, A.; Bhattacharyya, P.; Bhatia, D. Pomegranate exerts chemoprevention of experimentally induced mammary tumorigenesis by suppression of cell proliferation and induction of apoptosis. Nutr. Cancer 2015. [Google Scholar] [CrossRef]
- Matthews, J.; Gustafsson, J.A. Estrogen signaling: A subtle balance between ER alpha and ER beta. Mol. Interv. 2003, 3, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.P. The role of oestrogen in the pathogenesis of obesity, type 2 diabetes, breast cancer and prostate disease. Eur. J. Cancer Prev. 2010, 19, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Russo, I.H. Experimentally induced mammary tumors in rats. Breast Cancer Res. Treat. 1996, 39, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Kilańczyk, E.; Gwoździński, K.; Wilczek, E.; Filipek, A. Up-regulation of CacyBP/SIP during rat breast cancer development. Breast Cancer 2014, 21, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.D.; Davidson, N.E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Althuis, M.D.; Fergenbaum, J.H.; Garcia-Closas, M.; Brinton, L.A.; Madigan, M.P.; Sherman, M.E. Etiology of hormone receptor-defined breast cancer: A systematic review of the literature. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1558–1568. [Google Scholar]
- Korach, K.S.; Couse, J.F.; Curtis, S.W.; Washburn, T.F.; Lindzey, J.; Kimbro, K.S.; Eddy, E.M.; Migliaccio, S.; Snedeker, S.M.; Lubahn, D.B.; et al. Estrogen receptor gene disruption: Molecular characterization and experimental and clinical phenotypes. Recent Prog. Horm. Res. 1996, 51, 159–186. [Google Scholar] [PubMed]
- Saji, S.; Jensen, E.V.; Nilsson, S.; Rylander, T.; Warner, M.; Gustafsson, J.A. Estrogen receptors alpha and beta in the rodent mammary gland. Proc. Natl. Acad. Sci. USA 2000, 97, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Leygue, E.; Dotzlaw, H.; Watson, P.H.; Murphy, L.C. Altered estrogen receptor α and β messenger RNA expression during human breast tumorigenesis. Cancer Res. 1998, 58, 3197–3201. [Google Scholar] [PubMed]
- Lazennce, G.; Bresson, D.; Lucas, A.; Chauveau, C.; Vignon, F. ER beta inhibits proliferation and invasion of breast cancer cells. Endocrinology 2001, 142, 4120–4130. [Google Scholar]
- Jordan, V.C. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat. Rev. Cancer 2007, 7, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; DeCensi, A.; Arun, B.; Brown, P.H.; Castiglione, M.; Dunn, B.; Forbes, J.F.; Glaus, A.; Howell, A.; von Minckwitz, G.; et al. Preventive therapy for breast cancer: A consensus statement. Lancet Oncol. 2011, 12, 496–503. [Google Scholar] [CrossRef]
- Roger, P.; Sahla, M.E.; Mäkelä, S.; Gustafsson, J.A.; Baldet, P.; Rochefort, H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001, 61, 2537–2541. [Google Scholar] [PubMed]
- Brennan, K.R.; Brown, A.M.C. Wnt proteins in mammary development and cancer. J. Mammary Gland Biol. Neoplasia 2004, 9, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Incassati, A.; Chandramouli, A.; Eelkema, R.; Cowin, P. Key signaling nodes in mammary gland development and cancer: β-catenin. Breast Cancer Res. 2010, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Ikeda, S.; Ishizaki, Y.; Kurihara, T.; Tokumoto, N.; Iseki, M.; Arihiro, K.; Kataoka, T.; Okajima, M.; Asahara, T. Alterations and correlations of the components in the Wnt signaling pathway and its target genes in breast cancer. Oncol. Rep. 2005, 14, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Khramtsov, A.I.; Khramtsova, G.F.; Tretiakova, M.; Huo, D.; Olopade, O.I.; Goss, K.H. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 2010, 176, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- López-Knowles, E.; Zardawi, S.J.; McNeil, C.M.; Millar, E.K.; Crea, P.; Musgrove, E.A.; Sutherland, R.L.; O’Toole, S.A. Cytoplasmic localization of beta-catenin is a marker of poor outcome in breast cancer patients. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.P.; Gupta, S.D.; Rath, G.; Ralhan, R. Wnt signaling pathway in invasive ductal carcinoma of the breast: Relationship between beta-catenin, dishevelled and cyclin D1 expression. Oncology 2007, 73, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Karayiannakis, A.J.; Nakopoulou, L.; Gakiopoulou, H.; Keramopoulos, A.; Davaris, P.S.; Pignatelli, M. Expression patterns of beta-catenin in in situ and invasive breast cancer. Eur. J. Surg. Oncol. 2001, 27, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Currier, N.; Solomon, S.E.; Demicco, E.G.; Chang, D.L.; Farago, M.; Ying, H.; Dominguez, I.; Sonenshein, G.E.; Cardiff, R.D.; Xiao, Z.X.; et al. Oncogenic signaling pathways activated in DMBA-induced mouse mammary tumors. Toxicol. Pathol. 2005, 33, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pamukcu, R.; Thompson, W.J. β-Catenin signaling: Therapeutic strategies in oncology. Cancer Biol. Ther. 2002, 1, 621–625. [Google Scholar] [CrossRef]
- Bhatia, D.; Thoppil, R.J.; Mandal, A.; Samtani, K.A.; Darvesh, A.S.; Bishayee, A. Pomegranate Bioactive Constituents Suppress Cell Proliferation and Induce Apoptosis in an Experimental Model of Hepatocellular Carcinoma: Role of Wnt/β-Catenin Signaling Pathway. Evid. Based Complement. Altern. Med. 2013, 2013, 371813. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Li, L.; Celver, J.; Killian, C.; Kovoor, A.; Seeram, N.P. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. J. Agric. Food Chem. 2010, 58, 3965–3969. [Google Scholar] [CrossRef] [PubMed]
- Sadik, N.A.; Shaker, O.G. Inhibitory effect of a standardized pomegranate fruit extract on Wnt signalling in 1,2-dimethylhydrazine induced rat colon carcinogenesis. Dig. Dis. Sci. 2013, 58, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Neuman, E.; Ladha, M.H.; Lin, N.; Upton, T.M.; Miller, S.J.; DiRenzo, J.; Pestell, R.G.; Hinds, P.W.; Dowdy, S.F.; Brown, M.; et al. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol. Cell. Biol. 1997, 17, 5338–5347. [Google Scholar] [CrossRef] [PubMed]
- Caldon, C.E.; Sutherland, R.L.; Musgrove, E. Cell cycle proteins in epithelial cell differentiation: Implications for breast cancer. Cell Cycle 2010, 9, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.G.; Thompson, A.M. Cyclin D1 and breast cancer. Breast 2006, 15, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, T.; Morimura, K.; Yamashita, S.; Okochi, E.; Watanabe, N.; Ohta, T.; Ohki, M.; Fukushima, S.; Sugimura, T.; Ushijima, T. Etiology-specific gene expression profiles in rat mammary carcinomas. Cancer Res. 2002, 62, 3592–3597. [Google Scholar] [PubMed]
- Shan, L.; He, M.; Yu, M.; Qiu, C.; Lee, N.H.; Liu, E.T.; Snyderwine, E.G. cDNA microarray profiling of rat mammary gland carcinomas induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and 7,12-dimethylbenz[a]anthracene. Carcinogenesis 2002, 23, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Papaconstantinou, A.D.; Shanmugam, I.; Shan, L.; Schroeder, I.S.; Qiu, C.; Yu, M.; Snyderwine, E.G. Gene expression profiling in the mammary gland of rats treated with 7,12-dimethylbenz[a]anthracene. Int. J. Cancer 2006, 118, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tetsu, O.; McCormick, F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999, 398, 422–426. [Google Scholar] [PubMed]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.P.; Jiang, W.; Mo, H.; Bravo, L.; Froom, P.; Yu, W.; Harris, N.M.; Neeman, I.; Campbell, M.J. Possible synergistic prostate cancer suppression by anatomically discrete pomegranate fractions. Investig. New Drugs 2005, 23, 11–20. [Google Scholar] [CrossRef] [PubMed]
- De Kok, T.M.; van Breda, S.G.; Manson, M.M. Mechanisms of combined action of different chemopreventive dietary compounds: A review. Eur. J. Nutr. 2008, 47, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. Epigallocatechin 3-gallate and green tea catechins: United they work, divided they fail. Cancer Prev. Res. 2009, 2, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Bhatia, D.; Thoppil, R.J.; Darvesh, A.S.; Nevo, E.; Lansky, E.P. Pomegranate-mediated chemoprevention of experimental hepatocarcinogenesis involves Nrf2-regulated antioxidant mechanisms. Carcinogenesis 2011, 32, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, A.; Bishayee, A. Mechanism of Breast Cancer Preventive Action of Pomegranate: Disruption of Estrogen Receptor and Wnt/β-Catenin Signaling Pathways. Molecules 2015, 20, 22315-22328. https://doi.org/10.3390/molecules201219853
Mandal A, Bishayee A. Mechanism of Breast Cancer Preventive Action of Pomegranate: Disruption of Estrogen Receptor and Wnt/β-Catenin Signaling Pathways. Molecules. 2015; 20(12):22315-22328. https://doi.org/10.3390/molecules201219853
Chicago/Turabian StyleMandal, Animesh, and Anupam Bishayee. 2015. "Mechanism of Breast Cancer Preventive Action of Pomegranate: Disruption of Estrogen Receptor and Wnt/β-Catenin Signaling Pathways" Molecules 20, no. 12: 22315-22328. https://doi.org/10.3390/molecules201219853
APA StyleMandal, A., & Bishayee, A. (2015). Mechanism of Breast Cancer Preventive Action of Pomegranate: Disruption of Estrogen Receptor and Wnt/β-Catenin Signaling Pathways. Molecules, 20(12), 22315-22328. https://doi.org/10.3390/molecules201219853