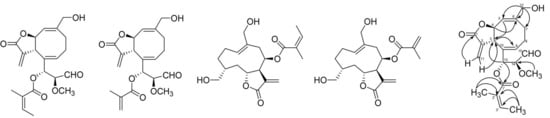
2. Results and Discussion
Dried
S. glabrescens was extracted with methanol. The crude methanol extract was subjected to liquid-liquid partition as well as a combination of several column chromatography steps to yield two new sesquiterpenoids
1 and
2 together with two known sesquiterepnoids
3 and
4 (
Figure 1).
Figure 1.
Chemical structures of compounds 1–4 and key HMBC (→) and NOESY (bold ↔) correlations for compound 1.
Figure 1.
Chemical structures of compounds 1–4 and key HMBC (→) and NOESY (bold ↔) correlations for compound 1.
Compound
1 was obtained as an amorphous solid. The HREIMS spectrum suggested a molecular formula of
1 as C
21H
26O
7. The
13C-NMR, DEPT, and HSQC spectra showed twenty-one carbon signals including three carbonyl carbons, eight olefinic carbons, three methylene carbons, four methine carbons, one methoxyl and two methyl groups. In
1H-
1H COSY spectrum, H-4 (δ 5.26) correlated with H-3 (δ 2.82) and H-5 (δ 5.09), and also H
2-8 (δ 2.62~2.73) correlated with methylene protons of H
2-7 (δ 2.06 and 2.78) and H-9 (δ 6.97). This data suggested that this compound has AMX and A
2M
2X spin systems. In HMBC spectrum, oxy-methylene protons H
2-12 (δ 4.36 and δ 4.39) correlated with C-5 (δ 129.4), C-6 (δ 142.3) and C-7 (δ 33.3), and H-3 (δ 2.82) correlated with C-9 (δ 159.7) and C-10 (δ 141.5) (
Figure 1 and
Table 1). These data indicated that compound
1 has eight-membered ring in the structure with two double bonds. The correlation between terminal methylene H
2-11 (δ 5.75 and 6.15) and a carbonyl carbon C-1 (δ 171.2) and C-3 (δ 51.9) in HMBC indicated that compound
1 is a bicycle [6.3.0]-γ-lactone having an exocyclic double bond in lactone ring. We also found the HMBC correlations between oxy-methine H-4 and C-6, and between H-5 and C-3. In
1H-
1H COSY spectrum, we found another AMX spin system from the correlations of H-14 (δ 3.94) with H-13 (δ 6.63) and H-15 (δ 9.43). From the coupling constant of H-15 (
J = 2.0 Hz) and chemical shift of C-15 (δ 196.8) and the HMBC correlations between H-15 (δ 9.43) and C-14 (δ 79.4), we identified the presence of an aldehyde group that is linked to C-14. In HMBC spectrum, we also found correlation between a methoxyl protons (δ 3.10) and C-14 (δ 79.4), and correlation between H-13 (δ 6.63) and C-3 (δ 51.9). The long range allylic coupling was observed between H-13 (dd, 8.4, 1.2 Hz) and H-9. These results indicated that an oxy-carbon C-13 is linked to the eight-membered ring at C-10. From the coupling constant between H-3 and H-4 (
J = 10.0 Hz,) we postulated the configurations of H-3 and H-4 based on Karplus relationship and the reported values of several bicycle [6.3.0]-γ-lactone derivatives (
Figure 1 and
Table 1) [
8]. Furthermore, the presence of 2-methylbut-2-enoyl group was recognized by
1H-
1H COSY correlation of an olefinic methine H-3' (δ 6.10) with methyl protons (δ 1.93) and the HMBC correlations of methyl protons (δ 1.88) with C-2' (δ 128.9) and C-1' (δ 168.4), and the correlations of methyl protons (δ 1.93) with C-2' (δ 128.9) and C-3' (δ 138.9). The HMBC correlation of an oxy-proton H-13 with carbonyl ester C-1' confirmed the esterification of 2-methylbut-2-enoyl group at C-13. We found NOESY correlations of H-13 (δ 6.63) with H-3 (δ 2.82) and H-11a (δ 6.15) that indicates the orientation of H-13 as β that is located close to H-3 and H-11a. NOESY correlations was also observed between H-9 (δ 6.97) and H-15 (δ 9.43) implying the orientation of methoxyl group as β. This orientation was confirmed by the coupling constant between H-13 and H-14 as 8.2 Hz. Thus, compound
1 was identified as 2-methylbut-2-enoic acid 1-(8-hydroxymethyl-3-methylene-2-oxo-2,3,3a,6,9a-hexahydro-cycloocta[b]furan-4-yl)-2-methoxy-3-oxo-propyl ester. This structure is new and we named compound
1 as siegenolide A.
Compound 2 was obtained as an amorphous solid. The HREIMS spectrum suggested a molecular formula as C20H24O7. The 13C-NMR, DEPT, and HSQC spectra showed similar signals as those of compound 1 except showing one terminal olefinic methylene signals of H2-3' (δ 5.65, 6.14) instead of the methyl protons (H3-4') of compound 1. The relative stereochemistry of compound 2 was determined by the analysis of NOESY spectra and coupling constants, which was same as compound 1. Thus, the structure of compound 2 was determined as 2-methyl-acrylic acid 1-(8-hydroxymethyl-3-methylene-2-oxo-2,3,3a,6,9a-hexahydro-cycloocta[b]furan-4-yl)-2-methoxy-3-oxo-propyl ester, which was a new structure and named as siegenolide B.
Compounds
3 and
4 were identified as 2-methylbut-2-enoic acid,2,3,3a,4,5,8,9,10,11,11a-decahydro-6,10-bis(hydroxymethyl)-3-methylene-2-oxocyclodeca[b]furan-4-yl ester (
3) and 2-methylacrylic acid, 2,3,3a,4,5,8,9,10,11,11a-decahydro-6,10-bis(hydroxymethyl)-3-methylene-2-oxocyclodeca[b]-furan-4-yl ester (
4), respectively, by comparison with the reported spectral data (
Figure 1) [
2,
9].
The four sesquiterpenoids
1–
4 were evaluated for their cytotoxic activity on human cancer cell lines such as MCF-7, AsPC-1, SW480, HCT 116, HepG2 and HeLa cells. Compounds
1–
4 showed differential cytotoxic effects on these cancer cell lines (
Table 2). All of them showed significant cytotoxicity against SW480 cell line, with IC
50 values of 1.8, 0.9, 5.2 and 3.8 μM, respectively. The cytotoxicity of compounds
3 and
4 against AsPC-1 cells was more potent (IC
50 values of 7.3 and 4.9 μM, respectively) than that of compounds
1 and
2 (IC
50 values 14.5 and 12.1 μM, respectively).
Table 1.
NMR Spectroscopic data (400 MHz, CD3OD) for siegenolides A (1) and B (2).
Table 1.
NMR Spectroscopic data (400 MHz, CD3OD) for siegenolides A (1) and B (2).
| Position | Siegenolide A (1) | Siegenolide B (2) |
|---|
| δC | δH (J in Hz) | HBMC a | δC | δH (J in Hz) | HBMC a |
|---|
| 1 | 171.2 | | | 171.3 | | |
| | 136.8 | | | 136.7 | | |
| 3 | 51.9 | 2.82, m | 9, 10 | 52.0 | 2.83, m | 9 |
| 4 | 75.7 | 5.26, t (10.0) | 6 | 75.7 | 5.33, t (10.0) | |
| 5 | 129.4 | 5.09, d (10.0) | 3 | 129.5 | 5.09, d (10.0) | 7, 12 |
| 6 | 142.3 | | | 142.2 | | |
| 7a | 33.3 | 2.78, m | | 33.5 | 2.78, m | |
| 7b | | 2.06, td (12.4, 2.0) | | | 2.06, td (12.4, 2.0) | |
| 8 | 28.4 | 2.62~2.73, m | | 28.5 | 2.74~2.68, m | |
| 9 | 159.7 | 6.97, dd (10.4, 7.6) | | 159.6 | 6.97, dd (10.4, 7.6) | |
| 10 | 141.5 | | | 141.6 | | |
| 11a | 121.5 | 6.15, d (3.2) | 1, 3 | 121.4 | 6.13, d (3.2) | 1, 3 |
| 11b | | 5.75, d (3.2) | 1, 3 | | 5.73, d (3.2) | 1 |
| 12a | 60.8 | 4.39, brd (13.2) | 5, 6, 7 | 61.0 | 4.39, brd (13.2) | 5, 6, 7 |
| 12b | | 4.36, brd (13.2) | 5, 6, 7 | | 4.33, brd (13.2) | 5, 6, 7 |
| 13 | 70.6 | 6.63, dd (8.4, 1.2) | 3, 1' | 71.6 | 6.56, dd (8.4, 1.6) | 1' |
| 14 | 79.4 | 3.94, dd (8.4, 2.0) | | 79.4 | 3.92, dd (8.4, 2.0) | 15 |
| 15 | 196.8 | 9.43, d (2.0) | 14 | 196.8 | 9.44, d (2.0) | 14 |
| 1' | 168.4 | | | 167.7 | | |
| 2' | 128.9 | | | 137.4 | | |
| 3' | 138.9 | 6.10, m | | 126.8 | 5.65, dd (3.2, 1.6) | |
| | | | | | 6.14, dd (3.2, 1.6) | |
| 14-OCH3 | 56.9 | 3.10, s | 14 | 57.0 | 3.09, s | 14 |
| 2'-Me | 20.7 | 1.88, pentet (1.6) | 1', 2' | 18.5 | 1.94, brs | 1', 2' |
| 3'-Me | 15.9 | 1.93, dq (7.2, 1.6) | 2', 3' | | | |
Table 2.
Cytotoxicity of compounds 1–4 against cancer cell lines.
Table 2.
Cytotoxicity of compounds 1–4 against cancer cell lines.
| Compounds | IC50 (μM) |
|---|
| MCF-7 | AsPC-1 | SW480 | HCT116 | HepG2 | HeLa |
|---|
| 1 | 9.5 ± 0.3 | 14.5 ± 0.9 | 1.8 ± 0.1 | 5.9 ± 0.2 | 20.2 ± 1.1 | 33.3 ± 2.3 |
| 2 | 8.7 ± 0.4 | 12.1 ± 0.2 | 0.9 ± 0.1 | 3.2 ± 0.3 | 9.9 ± 0.4 | 23.9 ± 1.2 |
| 3 | 9.7 ± 0.7 | 7.3 ± 0.5 | 5.2 ± 0.4 | 9.2 ± 0.6 | 14.4 ± 1.0 | 12.3 ± 0.7 |
| 4 | 12.7 ± 0.7 | 4.9 ± 0.2 | 3.8 ± 0.1 | 11.4 ± 0.8 | 27.8 ± 1.4 | 24.7 ± 0.9 |
| cisplatin | 13.0 ± 0.6 | 2.3 ± 0.2 | 4.8 ± 0.4 | 3.6 ± 0.1 | 5.9 ± 0.7 | 0.89 ± 0.1 |
Against HCT116 and HepG2 cells, compound
2 showed relatively high cytotoxicity, and against MCF-7 cells all compounds showed moderate cytotoxicity. All of these compounds displayed weak cytotoxicity against HeLa cells, with IC
50 values (12.3–33.3 μM) compared to that of cisplatin (0.9 μM). Cytotoxicity of sesquiterpenes with α, β-unsaturated lactone structure was well known. Compounds
1 and
2 are uncommon sesquiterpenoids with eight-membered ring and they also showed same type of cytotoxicity as reported [
10].
3. Experimental Section
3.1. General Experimental Procedures
UV spectra were recorded using an Ultraspec 4000 double beam spectrophotometer (Pharmacia Biotech, Cambridge, UK). 1D- and 2D-NMR spectra were obtained on a UNITY INOVA 400 spectrometer (Varian, Palo Alto, CA, USA). Mass spectra were determined on a JMS-AX505WA mass spectrometer (JEOL, Tokyo, Japan). Column chromatography was carried out over silica gel (40–60 μm, Merck, Merck, Kenilworth, NJ, USA), LiChroprep RP-C18 (40–60 μm, Merck) and µ-Bondapak C18 column (10 μm, 10 i.d. × 300 mm) (Waters Co., Milford, MA, USA). Fractions obtained from column chromatography were monitored by thin layer chromatography (TLC) (RP-C18 F254S and silica gel 60 F254, Merck).
3.2. Plant Material
The whole plant of Siegesbeckia glabrescens Makino (Compositae) was collected from Wan-Do, Jeolla-Namdo Province, Korea in November 2005 and authenticated by Prof. K. S. Yang at the College of Pharmacy, Sookmyung Women’s University (SMU). A voucher specimen (No. SPH 2005007) was deposited in the herbarium of SMU.
3.3. Extraction and Isolation
The air-dried material (5 kg) was reflux extracted with methanol (6 × 2 L) to yield after solvent removal a crude methanol extract (578 g), which was successively partitioned twice with EtOAc (3 L) and H2O (3 L). The EtOAc soluble fraction (368 g) was subjected to silica gel column chromatography (CC) (13 × 26 cm, 0.063–0.2 mm) eluting with a gradient mixture of CHCl3–MeOH (100:1, 70:1, 30:1, 20:1, 12:1, 5:1; 2 L each) to give 26 fractions. Fraction 9 (45 g, VR 5.5–6.0 L) was further fractionated by silica gel column with a gradient elution of CHCl3–MeOH (40:1 to 36:1, 2 L each) to afford Fr. 9-4 (3.4 g, VR 1.2–1.6 L). Fr. 9-4 was subjected to an ODS column (5 × 8 cm, 0.040–0.063 mm) eluting with MeOH–H2O (1:1) to afford cytotoxic Fr. 9-4-1 (400 mg, VR 1.2–1.4 L) which was subjected to Sephadex LH-20 CC (0.018–0.111 mm) eluted with 70% MeOH to yield pure compound 1 (18 mg, VR 230–290 mL). Fr. 9-4-1-1 (113.6 mg) was subjected to ODS column chromatography eluting with MeOH–H2O (1:1.6) to yield compound 2 (11.2 mg, VR 80–120 mL). Fr. 11 (4.0 g, VR 9.1–10.8 L) was separated by a silica gel column chromatography eluting with CHCl3–MeOH (40:1) to obtain cytotoxic Fr. 11-9 (234 mg, VR 2.8–3.2 L). Fr. 11-9 (234 mg) was purified by ODS column chromatography eluting with MeOH–H2O (1:3) to yield compounds 3 (40 mg, VR 120–145 mL) and 4 (11 mg, VR 210–240 mL). The purities of compounds 1–4 were higher than 95% as confirmed by HPLC chromatogram and 1H-NMR spectra.
Compound
1: amorphous solid;
: −96.4, (c 0.005, MeOH), UV (MeOH) λ
max (log ε) 244 (3.11), 235 (3.10) nm; IR (CaF
2, cm
−1) 3476, 2925, 1764, 1720, 1686.
1H- and
13C-NMR data, see
Table 1; HREIMS
m/z 390.1685 [M]
+ (calcd for C
21H
26O
7, 390.1678); EIMS
m/z 390 [M]
+.
Compound
2: amorphous solid;
: −5.7, (c 0.006, MeOH), UV (MeOH) λ
max (log ε) 253 (3.20), 245 (3.18) nm; IR (CaF
2, cm
−1) 3500, 2931, 1766, 1722, 1686, 1157.
1H- and
13C-NMR data, see
Table 1; HREIMS
m/z 376.1518 [M]
+ (calcd for C
20H
24O
7, 376.1522); EIMS
m/z 376 [M]
+.
3.4. Cytotoxicity Assay
The cytotoxicity of compounds
1–
4 against human breast cancer (MCF-7), pancreatic cancer (AsPC-1), colon cancers (SW480 and HCT 116), hepatoma (HepG2), and cervical carcinoma (HeLa) were assessed by the MTT method [
11]. Cells were plated at a density of 3000 cells/well in a 96 well plate. Cells were incubated with various concentrations of compounds
1–
4 for 3 days, and then treated with MTT (5 mg/mL) solution for 4 h and lysed with DMSO. Absorbance at 540 nm was measured by using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Cisplatin (purity > 98%) (Sigma, St. Louis, MO, USA) was used as a positive control.