Optimization of γ-Aminobutyric Acid Production by Lactobacillus plantarum Taj-Apis362 from Honeybees
Abstract
:1. Introduction
2. Results and Discussion
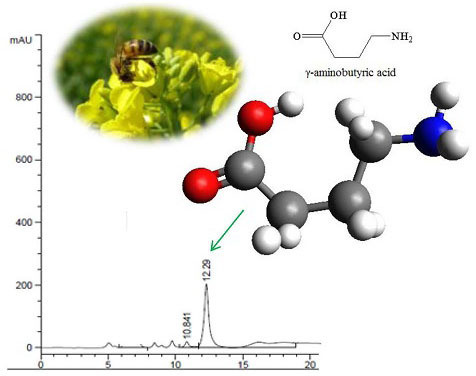
2.1. Evaluation of GABA-Producing Lactic Acid Bacteria


2.2. Characterization of Lactobacillus plantarum Taj-Apis362
2.3. Single Parameter
2.3.1. Effect of Culture Temperature on Growth Profile and GABA Production

2.3.2. Effect of Initial pH of the Culture Medium on Growth Profile and GABA Production

2.3.3. Effect of Initial Glutamic Acid Concentrations on Growth Profile and GABA Production

2.3.4. Effect of Incubation Time on Growth Profile and GABA Production

2.4. Analysis of Response Surface Methodology (RSM)
| Trials | Factor A Temperature (°C) | Factor B pH | Factor C Glutamic Acid (mM) | Factor D Time (h) | Actual GABA (mM) | Predicted GABA (mM) | Absolute Deviation |
|---|---|---|---|---|---|---|---|
| 1 | 33.75 | 4.875 | 462.5 | 36 | 4.198 ± 0.004 | 4.283 | 0.0202 |
| 2 | 41.25 | 4.875 | 462.5 | 36 | 5.914 ± 0.017 | 5.769 | 0.0245 |
| 3 | 33.75 | 5.625 | 462.5 | 36 | 5.087 ± 0.023 | 5.103 | 0.0031 |
| 4 | 41.25 | 5.625 | 462.5 | 36 | 4.937 ± 0.015 | 5.137 | 0.0405 |
| 5 | 33.75 | 4.875 | 587.5 | 36 | 4.697 ± 0.002 | 4.632 | 0.0138 |
| 6 | 41.25 | 4.875 | 587.5 | 36 | 3.811 ± 0.006 | 4.067 | 0.0672 |
| 7 | 33.75 | 5.625 | 587.5 | 36 | 5.325 ± 0.004 | 5.131 | 0.0364 |
| 8 | 41.25 | 5.625 | 587.5 | 36 | 3.302 ± 0.002 | 3.113 | 0.0572 |
| 9 | 33.75 | 4.875 | 462.5 | 60 | 5.351 ± 0.004 | 5.261 | 0.0168 |
| 10 | 41.25 | 4.875 | 462.5 | 60 | 5.517 ± 0.022 | 5.720 | 0.0368 |
| 11 | 33.75 | 5.625 | 462.5 | 60 | 6.766 ± 0.004 | 6.520 | 0.0364 |
| 12 | 41.25 | 5.625 | 462.5 | 60 | 5.741 ± 0.005 | 5.527 | 0.0373 |
| 13 | 33.75 | 4.875 | 587.5 | 60 | 5.952 ± 0.001 | 5.762 | 0.0319 |
| 14 | 41.25 | 4.875 | 587.5 | 60 | 4.466 ± 0.009 | 4.170 | 0.0663 |
| 15 | 33.75 | 5.625 | 587.5 | 60 | 6.833 ± 0.004 | 6.698 | 0.0198 |
| 16 | 41.25 | 5.625 | 587.5 | 60 | 3.730 ± 0.050 | 3.655 | 0.0201 |
| 17 | 30.0 | 5.25 | 525 | 48 | 5.495 ± 0.005 | 5.770 | 0.0500 |
| 18 | 45.0 | 5.25 | 525 | 48 | 4.218 ± 0.005 | 4.212 | 0.0014 |
| 19 | 37.5 | 4.50 | 525 | 48 | 3.897 ± 0.004 | 3.882 | 0.0038 |
| 20 | 37.5 | 6.00 | 525 | 48 | 3.903 ± 0.008 | 4.187 | 0.0728 |
| 21 | 37.5 | 5.25 | 400 | 48 | 5.632 ± 0.001 | 5.593 | 0.0069 |
| 22 | 37.5 | 5.25 | 650 | 48 | 3.762 ± 0.007 | 4.070 | 0.0819 |
| 23 | 37.5 | 5.25 | 525 | 24 | 5.638 ± 0.003 | 5.521 | 0.0208 |
| 24 | 37.5 | 5.25 | 525 | 72 | 6.653 ± 0.009 | 7.040 | 0.0582 |
| 25 | 37.5 | 5.25 | 525 | 48 | 6.422 ± 0.003 | 6.674 | 0.0392 |
| 26 | 37.5 | 5.25 | 525 | 48 | 6.693 ± 0.004 | 6.674 | 0.0028 |
| 27 | 37.5 | 5.25 | 525 | 48 | 6.883 ± 0.001 | 6.674 | 0.0304 |
| 28 | 37.5 | 5.25 | 525 | 48 | 6.618 ± 0.002 | 6.674 | 0.0085 |
| 29 | 37.5 | 5.25 | 525 | 48 | 6.607 ± 0.004 | 6.674 | 0.0101 |
| 30 | 37.5 | 5.25 | 525 | 48 | 6.818 ± 0.004 | 6.674 | 0.0211 |
2.4.1. Response Surface Methodology
| Source | SS | DF | MS | F Value | Prob ˃ F | |
|---|---|---|---|---|---|---|
| Model | 36.49708 | 14 | 2.606934 | 36.62527 | <0.0001 | significant |
| A | 3.641286 | 1 | 3.641286 | 51.15706 | <0.0001 | |
| B | 0.139047 | 1 | 0.139047 | 1.953495 | 0.1825 | |
| C | 3.477903 | 1 | 3.477903 | 48.86166 | <0.0001 | |
| D | 3.462349 | 1 | 3.462349 | 48.64314 | <0.0001 | |
| AB | 2.109061 | 1 | 2.109061 | 29.63056 | <0.0001 | |
| AC | 4.206169 | 1 | 4.206169 | 59.09320 | <0.0001 | |
| AD | 1.053595 | 1 | 1.053595 | 14.80214 | <0.0016 | |
| BC | 0.103610 | 1 | 0.103610 | 1.455634 | 0.2463 | |
| BD | 0.192077 | 1 | 0.192077 | 2.698520 | 0.1212 | |
| CD | 0.022921 | 1 | 0.022921 | 0.322022 | 0.5788 | |
| A2 | 4.853184 | 1 | 4.853184 | 68.18323 | <0.0001 | |
| B2 | 11.94087 | 1 | 11.94087 | 167.7594 | <0.0001 | |
| C2 | 5.816387 | 1 | 5.816387 | 81.71543 | <0.0001 | |
| D2 | 0.265332 | 1 | 0.265332 | 3.727700 | 0.0726 | |
| Residual | 1.067679 | 15 | 0.071179 | |||
| Lack of Fit | 0.931735 | 10 | 0.093173 | 3.426908 | 0.0932 | not significant |
| Pure Error | 0.135944 | 5 | 0.027189 | |||
| Cor Total | 37.56476 | 29 |

2.4.2. Verification of the Fitted Model and Optimum Point
| No. | Temperature (°C) | pH | Glutamic Acid (mM) | Time (h) | Actual GABA (mM) | Predicted GABA (mM) | Absolute Deviation |
|---|---|---|---|---|---|---|---|
| 1 | 36 | 5.31 | 497.97 | 60 | 7.15 ± 0.015 | 7.210 | 0.0084 |
| 2 | 37 | 5.16 | 462.50 | 60 | 6.94 ± 0.024 | 6.842 | 0.0141 |
| 3 | 37.5 | 5.31 | 514.88 | 48 | 6.57 ± 0.009 | 6.730 | 0.0243 |
| 4 | 37.5 | 5.33 | 462.50 | 48 | 6.48 ± 0.018 | 6.567 | 0.0134 |
3. Experimental Section
3.1. Isolation and Identification of GABA-Producing LAB
3.2. Culture Medium and Conditions
3.3. Evaluation of GABA-Producing LAB
3.4. Measurement of GABA Content
3.5. Characterization of Lactobacillus plantarum Taj-Apis362
3.6. Single Parameter Optimization
3.7. Experimental Design
3.8. Response Surface Methodology (RSM)
3.9. Verification of Estimated Data


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wong, T.; Guin, C.; Bottiglieri, T.; Snead, O.C. Gaba, γ-hydroxybutyric acid, and neurological disease. Ann. Neurol. 2003, 54, 3–12. [Google Scholar] [CrossRef]
- Miura, D.; Ito, Y.; Mizukuchi, A.; Kise, M.; Aoto, H.; Yagasaki, K. Hypocholesterolemic action of pre-germinated brown rice in hepatoma-bearing rats. Life Sci. 2006, 79, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Park, K.B.; Oh, S.H. Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour. Technol. 2007, 98, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, M.Y.; Ji, G.E.; Lee, Y.S.; Hwang, K.T. Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis gaba100. Int. J. Food Microbiol. 2009, 130, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Lin, H.T.V.; Tsai, G.J. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis fpa 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochem. 2013, 48, 559–568. [Google Scholar] [CrossRef]
- Hiraga, K.; Ueno, Y.; Sukontasing, S.; Tanasupawat, S.; Oda, K. Lactobacillus senmaizukei sp. Nov., isolated from japanese pickle. Int. J. Syst. Evol. Microbiol. 2008, 58, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Kimoto, H.; Someya, Y.; Furukawa, S.; Suzuki, I. Production of gamma-aminobutyric acid by cheese starters during cheese ripening. J. Dairy Sci. 1998, 81, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, S.; De Angelis, M.; Di Cagno, R.; Rizzello, C.; Coda, R.; Gobbetti, M. Synthesis of gamma-aminobutyric acid by lactic acid bacteria isolated from a variety of italian cheeses. Appl. Environ. Microbiol. 2007, 73, 7283–7290. [Google Scholar] [CrossRef] [PubMed]
- Komatsuzaki, N.; Shima, J.; Kawamoto, S.; Momose, H.; Kimura, T. Production of γ-aminobutyric acid (gaba) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol. 2005, 22, 497–504. [Google Scholar] [CrossRef]
- Ueno, Y.; Hayakawa, K.; Takahashi, S.; Oda, K. Purification and characterization of glutamate decarboxylase from Lactobacillus brevis ifo 12005. Biosci. Biotechnol. Biochem. 1997, 61, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Tajabadi, N.; Mardan, M.; Manap, M.Y.A.; Shuhaimi, M.; Meimandipour, A.; Nateghi, L. Detection and identification of Lactobacillus bacteria found in the honey stomach of the giant honeybee apis dorsata. Apidologie 2011, 42, 642–649. [Google Scholar] [CrossRef]
- Tajabadi, N.; Mardan, M.; Manap, M.Y.A.; Mustafa, S. Molecular identification of Lactobacillus spp. Isolated from the honey comb of the honey bee (apis dorsata) by 16s rrna gene sequencing. J. Apic. Res. 2013, 52, 235–241. [Google Scholar] [CrossRef]
- Kato, T.; Inuzuka, L.; Kondo, M.; Matsuda, T. Growth of nisin-producing lactococci in cooked rice supplemented with soybean extract and its application to inhibition of bacillus subtilis in rice miso. Biosci. Biotechnol. Biochem. 2001, 65, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, R.; Bajpai, V.K.; Baek, K.-H. Production of gaba (γ-aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiu, T.; Gao, D.; Cao, Y. Medium optimization for production of gamma-aminobutyric acid by Lactobacillus brevis ncl912. Amino Acids 2010, 38, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Lü, F.X.; Lu, Z.X.; Bie, X.M.; Jiao, Y.; Sun, L.J.; Yu, B. Production of γ-aminobutyric acid by streptococcus salivarius subsp. Thermophilus y2 under submerged fermentation. Amino Acids 2008, 34, 473–478. [Google Scholar]
- Binh, T.T.T.; Ju, W.-T.; Jung, W.-J.; Park, R.-D. Optimization of γ-amino butyric acid production in a newly isolated Lactobacillus brevis. Biotechnol. Lett. 2014, 36, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, Y.; Wei, Z.-Z.; Yuan, W.-X.; Li, Y.-L.; Zhang, C.-H.; Xue, X.-T.; Zhou, H.-J. Determination and comparison of γ-aminobutyric acid (gaba) content in pu-erh and other types of chinese tea. J. Agric. Food Chem. 2011, 59, 3641–3648. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, D.; Cao, Y.; Xu, H. A high γ-aminobutyric acid-producing Lactobacillus brevis isolated from chinese traditionalpaocai. Ann. Microbiol. 2008, 58, 649–653. [Google Scholar] [CrossRef]
- Cho, Y.R.; Chang, J.Y.; Chang, H.C. Production of gamma-aminobutyric acid (gaba) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J. Microbiol. Biotechnol. 2007, 17, 104–109. [Google Scholar] [PubMed]
- Tajabadi, N. Comparison of Lactic Acid Bacteria and Bifidobacteria from Honey Stomachs and Honeycombs of Giant Honeybee (Apis Dorsata) in Kedah and Terengganu, Malaysia. Master Thesis, Universiti Putra Malaysia, Serdang, Selangor, Malaysia, 09 December 2010. [Google Scholar]
- Di Cagno, R.; Mazzacane, F.; Rizzello, C.G.; De Angelis, M.; Giuliani, G.; Meloni, M.; De Servi, B.; Gobbetti, M. Synthesis of γ-aminobutyric acid (gaba) by Lactobacillus plantarum dsm19463: Functional grape must beverage and dermatological applications. Appl. Microbiol. Biotechnol. 2010, 86, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Komatsuzaki, N.; Nakamura, T.; Kimura, T.; Shima, J. Characterization of glutamate decarboxylase from a high gamma-aminobutyric acid (gaba)-producer, Lactobacillus paracasei. Biosci. Biotechnol. Biochem. 2008, 72, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Nakajima, I.; Fujita, Y.; Kobayashi, M.; Kimoto, H.; Suzuki, I.; Aso, H. Lactococcus lactis contains only one glutamate decarboxylase gene. Microbiology 1999, 145, 1375–1380. [Google Scholar] [CrossRef]
- Li, H.; Qiu, T.; Huang, G.; Cao, Y. Production of gamma-aminobutyric acid by Lactobacillus brevis ncl912 using fed-batch fermentation. Microb. Cell Factories 2010, 9, 85–92. [Google Scholar] [CrossRef]
- Li, H.; Cao, Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 2010, 39, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajabadi, N.; Ebrahimpour, A.; Baradaran, A.; Rahim, R.A.; Mahyudin, N.A.; Manap, M.Y.A.; Bakar, F.A.; Saari, N. Optimization of γ-Aminobutyric Acid Production by Lactobacillus plantarum Taj-Apis362 from Honeybees. Molecules 2015, 20, 6654-6669. https://doi.org/10.3390/molecules20046654
Tajabadi N, Ebrahimpour A, Baradaran A, Rahim RA, Mahyudin NA, Manap MYA, Bakar FA, Saari N. Optimization of γ-Aminobutyric Acid Production by Lactobacillus plantarum Taj-Apis362 from Honeybees. Molecules. 2015; 20(4):6654-6669. https://doi.org/10.3390/molecules20046654
Chicago/Turabian StyleTajabadi, Naser, Afshin Ebrahimpour, Ali Baradaran, Raha Abdul Rahim, Nor Ainy Mahyudin, Mohd Yazid Abdul Manap, Fatimah Abu Bakar, and Nazamid Saari. 2015. "Optimization of γ-Aminobutyric Acid Production by Lactobacillus plantarum Taj-Apis362 from Honeybees" Molecules 20, no. 4: 6654-6669. https://doi.org/10.3390/molecules20046654
APA StyleTajabadi, N., Ebrahimpour, A., Baradaran, A., Rahim, R. A., Mahyudin, N. A., Manap, M. Y. A., Bakar, F. A., & Saari, N. (2015). Optimization of γ-Aminobutyric Acid Production by Lactobacillus plantarum Taj-Apis362 from Honeybees. Molecules, 20(4), 6654-6669. https://doi.org/10.3390/molecules20046654