Inhibition of Oxidative Stress and Skin Aging-Related Enzymes by Prenylated Chalcones and Other Flavonoids from Helichrysum teretifolium
Abstract
:1. Introduction
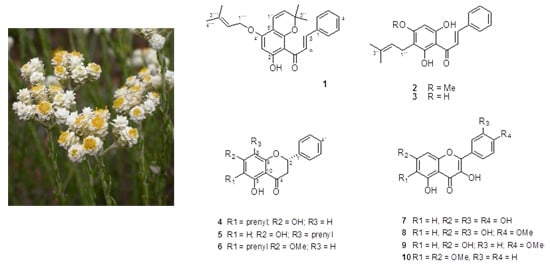
2. Results and Discussion
| No. | 1 | 2 | 3 | 4 | 5 | 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H | |
| 1 | 135.6 | 136.6 | 135.6 | |||||||||
| 2 | 128.2 | 7.58 dd , 7.6, 1.6 | 129.23 | 7.55 d , 6.2 | 128.3 | 7.53 dd , 1.6, 7.2 | 79.07 | 5.37 dd , 3.2, 13.2 | 79.1 | 5.30 dd , 2.5, 13.1 | 79.3 | 5.38 dd 3.0, 13.2 |
| 3 | 128.9 | 7.37 m | 129.88 | 7.29 m | 128.6 | 7.31 m | 43.44 | 3.05 dd , 13.2, 17.2 | 43.4 | 3.05 dd , 3.1, 17.2 | 43.5 | 3.05 dd 13.2, 17.1 |
| 2.79 dd , 3.2, 17.2 | 2.71 dd , 13.3, 17.2 | 2.78 dd 3.0, 17.1 | ||||||||||
| 4 | 130.0 | 7.37 m | 131.0 | 7.29 m | 129.7 | 7.31 m | 195.9 | - | 195.5 | 195.8 | ||
| 5 | 128.9 | 7.37 m | 129.9 | 7.29 m | 128.6 | 7.31 m | 161.2 | 161.1 | 161.3 | |||
| 6 | 128.2 | 7.58 dd , 7.6, 1.6 | 129.2 | 7.55 d , 6.2 | 128.3 | 7.53 dd , 1.6, 7.2 | 107.0 | 108.6 | 110.1 | |||
| 7 | 163.8 | 164.2 | 165.5 | |||||||||
| 8 | 95.5 | 5.99 s | 94.8 | 5.93 s | 91.0 | 6.07 s | ||||||
| 9 | 161.0 | 160.6 | 160.3 | - | ||||||||
| 10 | 102.9 | 102.3 | 102.9 | - | ||||||||
| 1' | 106.2 | - | 106.2 | 105.2 | 138.5 | 138.5 | 138.5 | - | ||||
| 2' | 167.3 | - | 164.7/164.6 | 163.4 | 126.1 | 7.30 m | 126.1 | 7.42 m | 126.1 | 7.37 m | ||
| 3' | 95.5 | 6.03 s | 109.2/109.1 | 106.7 | 128.8 | 7.30 m | 128.7 | 7.42 m | 128.9 | 7.37 m | ||
| 4' | 160.6 | - | 160.9 | 162.0 | 128.8 | 7.30 m | 128.7 | 7.42 m | 128.9 | 7.37 m | ||
| 5' | 103.3 | - | 91.6 | 6.05 s | 94.4 | 5.80 s | 128.8 | 7.30 m | 128.7 | 7.42 m | 128.9 | 7.37 m |
| 6' | 155.7 | - | 164.3/163.9 | 160.0 | 126.1 | 126.1 | 7.42 m | 126.1 | ||||
| α | 127.5 | 8.11 d 15.6 | 128.7/128.8 | 8.10/8.12 d , 15.7 | 127.9 | 8.07 d , 15.6 | ||||||
| β | 142.1 | 7.72 d 15.6 | 142.8/142.7 | 7.65 d , 15.7 | 141.7 | 7.68 d , 15.6 | ||||||
| CO | 192.8 | - | 193.8 | 192.9 | ||||||||
| 1'' | 117.0 | 6.58 d , 9.6 Hz | 22.0 | 3.22 d , 6.9 | 21.1 | 3.33 d , 7.2 | 20.9 | 3.21 d , 6.9 | 21.0 | 3.24 d , 6.8 | ||
| 2'' | 124.4 | 5.45 d , 9.6 Hz | 124.0 | 5.09 t , 6.9 | 121.4 | 5.28 t , 7.2 | 122.1 | 5.17 t , 6.9 | 122.2 | 5.16 tt , 6.8, 1.4 | ||
| 3'' | 77.9 | 136.6 | 135.7 | 132.4 | 131.7 | - | ||||||
| 4'' | 27.9 | 1.52 s | 26.0 | 1.48 s | 25.8 | 1.74 s | 25.6 | 1.63 s | 25.8 | 1.65 s | ||
| 5'' | 27.9 | 1.52 s | 17.9 | 1.60 s | 17.9 | 1.79 s | 17.7 | 1.72 s | 17.7 | 1.75 s | ||
| 1''' | 65.5 | 4.53 d , 6.4 | ||||||||||
| 2''' | 118.8 | 5.56 t , 6.4 | ||||||||||
| 3''' | 138.6 | - | ||||||||||
| 4''' | 25.8 | 1.77 s | ||||||||||
| 5''' | 18.3 | 1.72 s | ||||||||||
| 5-OH | - | 14.19 s | 13.61 s | 13.30 s | 12.36 s | 12.03 s | ||||||
| 7-OMe | 56.6 | 3.70 s | 55.8 | 3.81 s | ||||||||

| Sample | Automated Oxygen Radical Absorbance Capacity (ORAC μM TE/g) | FRAP | TEAC | ||
|---|---|---|---|---|---|
| ROO. (μM/g × 103) | OH. (μM/g × 106) | Prooxidant (μM/g) | μM AAE/g | μM TE/g | |
| HT | 1.313 ± 7.54 | 3.016 ± 5.90 | 4.163 ± 0.83 | 511.89 ± 4.61 | 1179.60 ± 8.20 |
| 1 | 2.833 ± 3.88 | 2.998 ± 1.67 | 2.036 ± 2.98 | ND | ND |
| 2 | 3.113 ± 17.59 | 2.910 ± 6.00 | 3.601 ± 2.23 | 619.91 ± 1.97 | 4170.66 ± 6.72 |
| 3 | 5.025 ± 6.16 | 3.771 ± 3.02 | 4.704 ± 0.27 | 817.94 ± 4.26 | 4529.01 ± 2.44 |
| 4 | 1.063 ± 33.50 | 2.918 ± 4.13 | 3.947 ± 0.29 | 7.052 ± 3.76 | 43.17 ± 6.26 |
| 5 | 0.856 ± 17.35 | 2.997 ± 0.36 | 2.971 ± 1.10 | 67.79 ± 14.27 | 204.15 ± 2.04 |
| 6 | 3.854 ± 5.14 | 2.955 ± 3.41 | 3.799 ± 0.60 | 104.09 ± 4.64 | 519.25 ± 3.66 |
| 7 | 17.836 ± 2.90 | 7.265 ± 0.71 | 4.361 ± 0.78 | 4816.31 ± 7.42 | 1361.70 ± 1.98 |
| 8 | 12.545 ± 5.07 | 6.779 ± 3.40 | 8.963 ± 2.79 | 3584.17 ± 0.54 | 1009.01 ± 1.98 |
| 9 | 10.491 ± 0.97 | 3.675 ± 1.40 | 3.790 ± 1.15 | 191.47 ± 1.39 | 261.30 ± 4.02 |
| 10 | 2.403 ± 2.50 | 2.909 ± 8.41 | 6.482 ± 1.55 | 544.60 ± 6.98 | 699.66 ± 2.28 |
| EGCG | 14.970 ± 5.53 | 3.911 ± 4.65 | 6.483 ± 1.19 | 3326.45 ± 5.76 | 11545.4 ± 17.28 |
| Sample | Inhibitory Activitis (IC50; μg/mL) * | ||
|---|---|---|---|
| Lipid Peroxidation | Tyrosinase | Elastase | |
| HT | 16.750 | 83.517 | 79.965 |
| 1 | >26.750 | >50 | >100 |
| 2 | >26.750 | >50 | >100 |
| 3 | 21.276 | >50 | >100 |
| 4 | >26.750 | >50 | >100 |
| 5 | >26.750 | >50 | >100 |
| 6 | 23.157 | >50 | >100 |
| 7 | 2.931 | 8.092 | 43.342 |
| 8 | 6.449 | 27.573 | >86.548 |
| 9 | 10.520 | 29.571 | >100 |
| 10 | 10.720 | 38.062 | >100 |
| EGCG | 0.929 | NA | NA |
| Kojic acid | NA | 3.425 | NA |
| Oleanolic acid | NA | NA | 9.806 |
3. Experimental Section
3.1. General Information
3.2. Preparation of Plant Extracts
3.3. Extraction and Purification of Chemical Constituents
3.4. Antioxidant Assays
3.4.1. Ferric-Ion Reducing Antioxidant Power Assay (FRAP)
3.4.2. Automated Oxygen Radicals Absorbance Capacity (ORAC) Assay
3.4.3. Trolox Equivalent Absorbance Capacity (TEAC) Assay
3.4.4. Inhibition of Fe (II)-Induced Microsomal Lipid Peroxidation Assay
3.4.5. Tyrosinase Enzyme Assay
3.4.6. Elastase Inhibition Assay
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Zhang, S.; Dong, Z.; Peng, Z.; Lu, F. Anti-aging effect of adipose-derived stem cells in a mouse model of skin aging induced by d-Galactose. PLoS ONE 2014, 9, e97573. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.R.; Kim, D.H.; Kim, S.R.; An, H.J.; Lee, E.K.; Tanaka, T.; Kim, N.D.; Tokozawa, T.; Park, J.N.; Chung, H.Y. Anti-wrinkle effect of magnesium lithospermate B from Salvia miltiorrhiza BUNGE: Inhibition of MMPs via NF-kB signaling. PLoS ONE 2014, 9, e102689. [Google Scholar] [CrossRef] [PubMed]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanism and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chompo, J.; Upadhyay, A.; Fukuta, M.; Tawata, S. Effect of Alpinia zerumbet components on antioxidant and skin disease-related enzymes. BMC Complement. Altern. Med. 2012, 12, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In vitro determination of the anti-aging potential of four southern African medicinal plants. BMC Complement. Altern. Med. 2013, 13, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. Fatty acid oxidation and isoprostanes: Oxidative strain and oxidative stress. Prostaglandins Leukot. Essent Fatty Acids 2010, 82, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Schrader, M.; Fahimi, H. Peroxisomes and oxidative stress. Biochim. Biophys. Acta 2006, 1763, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S. An update review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Wangthong, S.; Tonsiripakdee, I.; Monhaphol, T.; Nonthabenjawan, R.; Wanichwecharungruang, P.S. Coat TLC developing technique for tyrosinase inhibitor detection. Biomed. Chromatogr. 2007, 21, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, P.; Manning, J. Cape Plants: A conspectus of the Cape flora of South Africa, 9 ed.; National Botanical Institute: Pretoria, South Africa; Missouri Botanical Garden, St Louis, MO, USA, 2002; pp. 366–368. [Google Scholar]
- Lourens, A.C.U.; Viljoen, A.M.; van Heerden, F.R. South African Helichrysum species: A review of the traditional uses, biological activity and phytochemistry. J. Ethnopharmacol. 2008, 119, 630–650. [Google Scholar] [CrossRef] [PubMed]
- Viegas, D.A.; Palmeira-de-Oliveira, A.; Salgueiro, L. Helichrysum italicum: From traditional use to scientific data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Narasimhule, M.; Li, X.; Shim, J; Lee, Y. New synthetic routes to biological interesting geranylated acetophenones from Melicope semecarpifolia and their unnatural prenylated and fernesylated derivatives. Bull. Korean Chem. Soc. 2010, 31, 664–669. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; Hoffmann, E.; Mahanta, P.K.; Dorner, W. Neue diterpene und sesquiterpene aus südafrikanischen Helichrysum-arten. Phytochemistry 1978, 17, 1917–1922. [Google Scholar] [CrossRef]
- Bohlmann, F.; Ferdinand, Z.J.; Mahanta, P.K.; Pradip, K. New chalcone derivatives and humulone-like compounds from Helichrysum species. Phytochemistry 1979, 18, 1036–1042. [Google Scholar]
- Jakupovic, J.; Zdero, C.; Grenz, M.; Tsichritzis, F.; Lehmann, L.; Hashemi-Nejad, S.M.; Bohlmann, F. Twenty-one acylphloroglucinol derivatives and further constituents from south African Helichrysum species. Phytochemistry 1989, 28, 1119–1131. [Google Scholar] [CrossRef]
- Braz, F.R.; Gottlieb, O.R.; Mourao, A.P. Chemistry of Brazilian leguminosae 47 Stilbene and two flavanones from Derris rariflora. Phytochemistry 1975, 14, 261–264. [Google Scholar] [CrossRef]
- Prokopenko, A.P.; Spiridonov, V.N.; Litvinenko, V.I.; Chernobai, V.T. Flavonoid aglycones from Helichrysum arenarium racemes. Khimiya Prirodnykh Soedinenii 1972, 5, 649–650. [Google Scholar]
- Servettaz, O.; Colombo, M.L.; de Bernardi, M.; Uberti, E.; Vidari, G.; Vita-Finzi, P. Flavonol glycosides from Dryas octopetala. J. Nat. Prod. 1984, 47, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Gowsala, P.; Uvais, M.S. Flavonoids of some Dilleniacea species. Phytochemistry 1975, 14, 1127–1128. [Google Scholar] [CrossRef]
- Donald, B.; Stierle, A.A.; Larse, R.D. Terpenoid and flavone constituents of Polemonium viscosum. Phytochemistry 1988, 27, 517–522. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Structure-activity relationship of flavonoids in the cellular antioxidant activity assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationship. J. Nutr. Biochem. 2002, 13, 572–284. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 1–16. [Google Scholar]
- Narayanaswamy, N.; Duraisamy, A.; Balakrishnan, K.P. Screening of some medicinal plants for their Antityrosinase and Antioxidant activities. Int. J. PharmTech Res. 2011, 3, 1107–1112. [Google Scholar]
- Masuda, M.; Murata, K.; Fukuhama, A.; Naruto, S.; Fujita, T.; Uwaya, A.; Isami, F.; Matsuda, H. Inhibitory effects of constituents of Morinda atrifolia seeds on elastase and tyrosinase. J. Nat. Med. 2009, 63, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Tan, H.; Chen, J.; Wang, M. Characterisation of tyrosinase inhibitors in the twigs of Cudranis tricuspidata and their structure-activity relationship study. Fitoterapia 2013, 84, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modifies version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 238, 70–76. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampschwoodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (ORACFL) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Prior, R.L. Measurement of oxygen radical absorbance capacity in biological samples. Methods Enzymol. 1998, 299, 50–62. [Google Scholar]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C.A. Screening of dietary carotenoid-rich fruit extracts for antioxidant activities applying ABTS radical cation decolorisation assay. Methods Enzymol. 1999, 299, 379–389. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Snijman, P.W.; Joubert, E.; Ferreira, D.; Li, X.-C.; Ding, Y.; Green, I.R.; Gelderblom, W.C.A. Antioxidant activity of the dihydrochalcones aspalathin and nothofagin and their corresponding flavones in relation to other rooibos (Aspalathus linearis) flavonoids, epigallocatechin gallate, and Trolox. J. Agric. Food Chem. 2009, 57, 6678–6684. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, A.K.; Pandey, B. Screening of plant parts for anti-tyrosinase activity by tyrosinase assay using mushroom tyrosinase. Indian J. Sci. Res. 2014, 4, 134–139. [Google Scholar]
- Sample Availability: Samples of the compounds 3, 7 and 8 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popoola, O.K.; Marnewick, J.L.; Rautenbach, F.; Ameer, F.; Iwuoha, E.I.; Hussein, A.A. Inhibition of Oxidative Stress and Skin Aging-Related Enzymes by Prenylated Chalcones and Other Flavonoids from Helichrysum teretifolium. Molecules 2015, 20, 7143-7155. https://doi.org/10.3390/molecules20047143
Popoola OK, Marnewick JL, Rautenbach F, Ameer F, Iwuoha EI, Hussein AA. Inhibition of Oxidative Stress and Skin Aging-Related Enzymes by Prenylated Chalcones and Other Flavonoids from Helichrysum teretifolium. Molecules. 2015; 20(4):7143-7155. https://doi.org/10.3390/molecules20047143
Chicago/Turabian StylePopoola, Olugbenga K., Jeanine L. Marnewick, Fanie Rautenbach, Farouk Ameer, Emmanuel I. Iwuoha, and Ahmed A. Hussein. 2015. "Inhibition of Oxidative Stress and Skin Aging-Related Enzymes by Prenylated Chalcones and Other Flavonoids from Helichrysum teretifolium" Molecules 20, no. 4: 7143-7155. https://doi.org/10.3390/molecules20047143
APA StylePopoola, O. K., Marnewick, J. L., Rautenbach, F., Ameer, F., Iwuoha, E. I., & Hussein, A. A. (2015). Inhibition of Oxidative Stress and Skin Aging-Related Enzymes by Prenylated Chalcones and Other Flavonoids from Helichrysum teretifolium. Molecules, 20(4), 7143-7155. https://doi.org/10.3390/molecules20047143