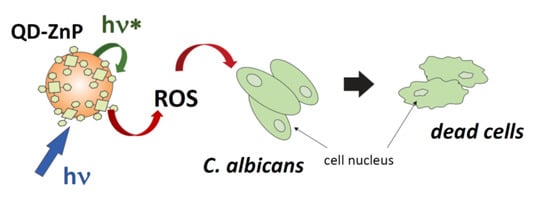
Comparative Study on the Efficiency of the Photodynamic Inactivation of Candida albicans Using CdTe Quantum Dots, Zn(II) Porphyrin and Their Conjugates as Photosensitizers
Abstract
:1. Introduction

2. Results and Discussion
2.1. Structural and Optical Characterization of the QDs, ZnP and QDs-ZnP


2.2. Spectroscopic Studies of the QD-ZnP Conjugates

2.3. Indirect Detection of ROS

2.4. Cytotoxicity Assays of the PSs in Fibroblast Cells

2.5. Photodynamic Inactivation Assays for Candida albicans

3. Experimental Section
3.1. Synthesis of CdTe QDs and ZnP
3.2. Characterization of the Systems
3.3. Characterization of QDs-ZnP Conjugates
3.4. Indirect Detection of ROS by the Oxidation of Nitrotetrazolium Blue (NBT)
3.5. Cytotoxicity of QDs and ZnP against Fibroblast Cells in the Presence and Absence of Radiation
3.6. Photodynamic Inactivation of Candida albicans
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tanaka, M.; Kinoshita, M.; Yoshihara, Y.; Shinomiya, N.; Seki, S.; Nemoto, K.; Hirayama, T.; Dai, T.; Huang, L.; Hamblin, M.R.; et al. Optimal photosensitizers for photodynamic therapy of infections should kill bacteria but spare neutrophils. Photochem. Photobiol. 2012, 88, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Yaghini, E.; Seifalian, A.M.; MacRobert, A.J. Quantum dots and their potential biomedical applications in photosensitization for photodynamic therapy. Nanomedicine 2009, 4, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Simplicio, F.I.; Maionchi, F.; Hioka, N. Terapia fotodinâmica: Aspectos farmacológicos, aplicações e avanços recentes no desenvolvimento de medicamentos. Quím. Nova 2002, 25, 801–807. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Lee, Y.-M.; Zhao, D.; Mak, N.-K.; Wong, R.N.-S.; Chan, W.-H.; Cheung, N.-H. Quantum dot-mediated photoproduction of reactive oxygen species for cancer cell annihilation. Photochem. Photobiol. 2010, 86, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Juzenas, P.; Chen, W.; Sun, Y.-P.; Coelho, M.A.N.; Generalov, R.; Generalova, N.; Christensen, I.L. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1600–1614. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.M.; Trzoss, M.; Shi, L.; Kong, X.; Selke, M.; Jung, M.E.; Weiss, S. Singlet oxygen production by peptide-coated quantum dot-photosensitizer conjugates. J. Am. Chem. Soc. 2007, 129, 6865–6871. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.E.D.H. Terapia fotodinâmica: Princípios, potencial de aplicação e perspectivas. Quím. Nova 2000, 23, 237–243. [Google Scholar] [CrossRef]
- Soukos, N.S.; Goodson, J.M. Photodynamic therapy in the control of oral biofilms. Periodontology 2000 2011, 55, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Cormick, M.P.; Alvarez, M.G.; Rovera, M.; Durantini, E.N. Photodynamic inactivation of Candida albicans sensitized by tri- and tetra-cationic porphyrin derivatives. Eur. J. Med. Chem. 2009, 44, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Caminos, D.A.; Spesia, M.B.; Pons, P.; Durantini, E.N. Mechanisms of Escherichia coli photodynamic inactivation by an amphiphilic tricationic porphyrin and 5,10,15,20-tetra(4-N,N,N-trimethylammoniumphenyl) porphyrin. Photochem. Photobiol. Sci. 2008, 7, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, S.; Aalders, M.; van Marle, J. Mechanistic study of the photodynamic inactivation of Candida albicans by a cationic porphyrin. Antimicrob. Agents Chemother. 2005, 49, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Benov, L.; Craik, J.; Batinic-Haberle, I. The potential of Zn (II) N-alkylpyridylporphyrins for anticancer therapy. Anti-Cancer Agent Med. Chem. 2011, 11, 233–241. [Google Scholar] [CrossRef]
- Benov, L.; Craik, J.; Batinic-Haberle, I. Protein damage by photo-activated Zn(II) N-alkylpyridylporphyrins. Amino Acids 2012, 42, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.L.; Bhaumik, J.; Diers, J.R.; Mroz, P.; Hamblin, M.R.; Bocian, D.F.; Lindsey, J.S.; Holten, D. Photophysical characterization of imidazolium-substituted Pd(II), In(III), and Zn(II) porphyrins as photosensitizers for photodynamic therapy. J. Photochem. Photobiol. A Chem. 2008, 200, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Odeh, A.M.; Craik, J.D.; Ezzeddine, R.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L.T. Targeting mitochondria by Zn (II) N-alkylpyridylporphyrins: The impact of compound sub-mitochondrial partition on cell respiration and overall photodynamic efficacy. PLos ONE 2014, 9, e108238. [Google Scholar] [CrossRef] [PubMed]
- Benov, L.; Batinić-Haberle, I.; Spasojević, I.; Fridovich, I. Isomeric N-alkylpyridylporphyrins and their Zn(II) complexes: Inactive as SOD mimics but powerful photosensitizers. Arch. Biochem. Biophys. 2002, 402, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ezzeddine, R.; Al-Banaw, A.; Tovmasyan, A.; Craik, J.D.; Batinic-Haberle, I.; Benov, L.T. Effect of molecular characteristics on cellular uptake, subcellular localization, and phototoxicity of Zn(ii) N-alkylpyridylporphyrins. J. Biol. Chem. 2013, 288, 36579–36588. [Google Scholar] [CrossRef] [PubMed]
- Pavani, C.; Uchoa, A.F.; Oliveira, C.S.; Iamamoto, Y.; Baptista, M.S. Effect of zinc insertion and hydrophobicity on the membrane interactions and PDT activity of porphyrin photosensitizers. Photochem. Photobiol. Sci. 2009, 8, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Pavani, C.; Iamamoto, Y.; Baptista, M.S. Mechanism and efficiency of cell death of type ii photosensitizers: Effect of zinc chelation. Photochem. Photobiol. 2012, 88, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Biju, V.; Itoh, T.; Anas, A.; Sujith, A.; Ishikawa, M. Semiconductor quantum dots and metal nanoparticles: Syntheses, optical properties, and biological applications. Anal. Bioanal. Chem. 2008, 391, 2469–2495. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, J.-F.; Won, N.; Jin, H.; Kim, S.; Chen, J.-Y. Quantum dot-aluminum phthalocyanine conjugates perform photodynamic reactions to kill cancer cells via fluorescence resonance energy transfer. Nanoscale Res. Lett. 2012, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morosini, V.; Bastogne, T.; Frochot, C.; Schneider, R.; François, A.; Guillemin, F.; Barberi-Heyob, M. Quantum dot-folic acid conjugates as potential photosensitizers in photodynamic therapy of cancer. Photochem. Photobiol. Sci. 2011, 10, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Samia, A.C.S.; Chen, X.; Burda, C. Semiconductor quantum dots for photodynamic therapy. J. Am. Chem Soc. 2003, 125, 15736–15737. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, J.-Y.; Idowu, M.; Nyokong, T. Generation of singlet oxygen via the composites of water-soluble thiol-capped cdte quantum dots sulfonated aluminum phthalocyanines. J. Phys. Chem. B 2008, 112, 4465–4469. [Google Scholar] [CrossRef] [PubMed]
- Fontes, A.; Lira, R.B.D.; Seabra, M.A.B.L.; Silva, T.G.D.; Neto, A.G.D.C.; Santos, B.S. Quantum dots in biomedical research. In Biomedical Engineering—Technical Applications in Medicine; Hudak, R., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 12. [Google Scholar] [CrossRef]
- Farias, P.; Santos, B.; Menezes, F.; Cesar, C.; Fontes, A.; Lima, P.; Barjas-Castro, M.; Castro, V.; Ferreira, R. CdS Quantum dots, efficient fluorescent markers for red cells. J. Microsc. 2005, 219, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Liu, J.; Haley, K.N.; Treadway, J.A.; Larson, J.P.; Ge, N.; Peale, F.; Bruchez, M.P. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 2003, 21, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, K.; Xie, R.; Xie, J.; Yan, Y.; Cheng, Z.; Peng, X.; Chen, X. In vivo tumor-targeted fluorescence imaging using near-infrared non-cadmium quantum dots. Bioconjugate Chem. 2010, 21, 604–609. [Google Scholar] [CrossRef]
- Bagalkot, V.; Zhang, L.; Levy-Nissenbaum, E.; Jon, S.; Kantoff, P.W.; Langer, R.; Farokhzad, O.C. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett. 2007, 7, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.J.; Hollmann, C.A.; Zhang, Z.; Suffern, D.; Bradforth, S.E.; Dimitrijevic, N.M.; Minarik, W.G.; Nadeau, J.L. Photophysics of dopamine-modified quantum dots and effects on biological systems. Nat. Mater. 2006, 5, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P.; Gu, W.; Larabell, C. Quantum dots as cellular probes. Ann. Rev. Biomed. Eng. 2005, 7, 55–76. [Google Scholar] [CrossRef]
- Tekdaş, D.A.; Durmuş, M.; Yanık, H.; Ahsen, V. Photodynamic therapy potential of thiol-stabilized CdTe quantum dot-group 3A phthalocyanine conjugates (QD-Pc). Spectrochim. Acta Part. A 2012, 93, 313–320. [Google Scholar] [CrossRef]
- Jhonsi, M.A.; Renganathan, R. Investigations on the photoinduced interaction of water soluble thioglycolic acid (TGA) capped CdTe quantum dots with certain porphyrins. J. Colloid Interface Sci. 2010, 344, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Zhongxin, L.; Lun, M.; Marius, H.; Wei, C. Interaction of porphyrins with CdTe quantum dots. Nanotechnology 2011, 22, 195501. [Google Scholar] [CrossRef] [PubMed]
- Rakovich, A.; Savateeva, D.; Rakovich, T.; Donegan, J.; Rakovich, Y.; Kelly, V.; Lesnyak, V.; Eychmüller, A. CdTe quantum dot/dye hybrid system as photosensitizer for photodynamic therapy. Nanoscale Res. Lett. 2010, 5, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.T.; Prates, R.A.; Sabino, C.P.; Fuchs, B.B.; Tegos, G.P.; Mylonakis, E.; Hamblin, M.R.; Ribeiro, M.S. Antimicrobial photodynamic inactivation inhibits Candida albicans virulence factors and reduces in vivo pathogenicity. Antimicrob. Agents Chemother. 2013, 57, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.M.; da Silva, D.L.; Sousa, G.R.; Amorim, J.C.F.; de Resende, M.A.; Pinotti, M.; Cisalpino, P.S. In vitro photodynamic inactivation of Candida spp. growth and adhesion to buccal epithelial cells. J. Photochem. Photobiol. B Biol. 2009, 94, 65–70. [Google Scholar] [CrossRef]
- Dai, T.; Fuchs, B.B.; Coleman, J.J.; Prates, R.A.; Astrakas, C.; St. Denis, T.G.; Ribeiro, M.S.; Mylonakis, E.; Hamblin, M.R.; Tegos, G.P. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front. Microbiol. 2012, 3, 120. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, A.; Zaragoza, O.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M. Pharmacotherapy of yeast infections. Expert Opin. Pharmacother. 2008, 9, 2801–2816. [Google Scholar] [CrossRef] [PubMed]
- Brion, L.P.; Uko, S.E.; Goldman, D.L. Risk of resistance associated with fluconazole prophylaxis: Systematic review. J. Infect. 2007, 54, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Oriel, S.; Nitzan, Y. Photoinactivation of Candida albicans by its own endogenous porphyrins. Curr. Microbiol. 2010, 60, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.J.; Cormick, M.P.; Milanesio, M.E.; Durantini, E.N. The photodynamic activity of a novel porphyrin derivative bearing a fluconazole structure in different media and against Candida albicans. Dyes Pigments 2010, 87, 234–240. [Google Scholar] [CrossRef]
- Walther, J.; Bröcker, M.J.; Wätzlich, D.; Nimtz, M.; Rohde, M.; Jahn, D.; Moser, J. Protochlorophyllide: A new photosensitizer for the photodynamic inactivation of Gram-positive and Gram-negative bacteria. FEMS Microbiol. Lett. 2009, 290, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, M.; Huang, Y.-Y.; Landi, G.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation with decacationic functionalized fullerenes: Oxygen-independent photokilling in presence of azide and new mechanistic insights. Free Radic. Biol. Med. 2015, 79, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, F.P.; Felgenträger, A.; Bäumler, W.; Maisch, T. Fungicidal photodynamic effect of a twofold positively charged porphyrin against Candida albicans planktonic cells and biofilms. Future Microbiol. 2013, 8, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Rosseti, I.; Chagas, L.; Costa, M. Photodynamic antimicrobial chemotherapy (PACT) inhibits biofilm formation by Candida albicans, increasing both ROS production and membrane permeability. Lasers Med. Sci. 2014, 29, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Powder Diffraction Standards. Powder Diffraction File Search Manual; JCPDS: Swarthmore, PA, USA, 2009; Index card: 00-015-0770. [Google Scholar]
- Frasco, M.; Vamvakaki, V.; Chaniotakis, N. Porphyrin decorated CdSe quantum dots for direct fluorescent sensing of metal ions. J. Nanopart Res. 2010, 12, 1449–1458. [Google Scholar] [CrossRef]
- Silva, F.O.; de Souza Viol, L.C.; Ferreira, D.L.; Alves, J.L.A.; Schiavon, M.A. O estado da arte da síntese de semicondutores nanocristalinos coloidais. Quim. Nova 2010, 33, 1933–1939. [Google Scholar] [CrossRef]
- Rogach, A.L.; Franzl, T.; Klar, T.A.; Feldmann, J.; Gaponik, N.; Lesnyak, V.; Shavel, A.; Eychmüller, A.; Rakovich, Y.P.; Donegan, J.F. Aqueous synthesis of thiol-capped CdTe nanocrystals: State-of-the-art. J. Phys. Chem. C 2007, 111, 14628–14637. [Google Scholar] [CrossRef]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Photochemistry of water-soluble porphyrins: Comparative study of isomeric tetrapyridyl- and tetrakis(N-methylpyridiniumyl)porphyrins. Inorg. Chem. 1984, 23, 2453–2459. [Google Scholar] [CrossRef]
- Kowerko, D.; Schuster, J.; Amecke, N.; Abdel-Mottaleb, M.; Dobrawa, R.; Wurthner, F.; von Borczyskowski, C. FRET and ligand related NON-FRET processes in single quantum dot-perylene bisimide assemblies. Phys. Chem. Chem. Phys. 2010, 12, 4112–4123. [Google Scholar] [CrossRef] [PubMed]
- Blaudeck, T.; Zenkevich, E.I.; Cichos, F.; von Borczyskowski, C. Probing wave functions at semiconductor quantum-dot surfaces by non-FRET photoluminescence quenching. J. Phys. Chem. C 2008, 112, 20251–20257. [Google Scholar] [CrossRef]
- Clapp, A.R.; Pons, T.; Medintz, I.L.; Delehanty, J.B.; Melinger, J.S.; Tiefenbrunn, T.; Dawson, P.E.; Fisher, B.R.; O’Rourke, B.; Mattoussi, H. Two-photon excitation of quantum-dot-based fluorescence resonance energy transfer and its applications. Adv. Mater. 2007, 19, 1921–1926. [Google Scholar] [CrossRef]
- Medintz, I.L.; Clapp, A.R.; Melinger, J.S.; Deschamps, J.R.; Mattoussi, H. A reagentless biosensing assembly based on quantum dot-donor Förster resonance energy transfer. Adv. Mater. 2005, 17, 2450–2455. [Google Scholar] [CrossRef]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Mattoussi, H. Quantum dot-based resonance energy transfer and its growing application in biology. Phys. Chem. Chem. Phys. 2009, 11, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Benov, L. Photodynamic therapy: Current status and future directions. Med. Princ. Pract. 2015, 24, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Garcez, A.S.; Nunez, S.C.; Baptista, M.S.; Daghastanli, N.A.; Itri, R.; Hamblin, M.R.; Ribeiro, M.S. Antimicrobial mechanisms behind photodynamic effect in the presence of hydrogen peroxide. Photochem. Photobiol. Sci. 2011, 10, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J. Investig. Dermatol. 2009, 130, 259–269. [Google Scholar] [CrossRef]
- Lavi, R.; Shainberg, A.; Friedmann, H.; Shneyvays, V.; Rickover, O.; Eichler, M.; Kaplan, D.; Lubart, R. Low energy visible light induces reactive oxygen species generation and stimulates an increase of intracellular calcium concentration in cardiac cells. J. Biol. Chem. 2003, 278, 40917–40922. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Tenório, D.P.L.A.; Andrade, C.G.; Cabral Filho, P.E.; Sabino, C.P.; Kato, I.T.; Carvalho, L.B., Jr.; Alves, S., Jr.; Ribeiro, M.S.; Fontes, A.; Santos, B.S. CdTe quantum dots conjugated to concanavalin A as potential fluorescent molecular probes for saccharides detection in Candida albicans. J. Photochem. Photobiol. B Biol. 2015, 142, 237–243. [Google Scholar] [CrossRef]
- Andrade, C.G.; Cabral Filho, P.E.; Tenório, D.P.; Santos, B.S.; Beltrão, E.I.; Fontes, A.; Carvalho, L.B., Jr. Evaluation of glycophenotype in breast cancer by quantum dot-lectin histochemistry. Int. J. Nanomed. 2013, 8, 4623. [Google Scholar]
- Rebouças, J.S.; de Carvalho, M.E.M.D.; Idemori, Y.M. Perhalogenated 2-pyridylporphyrin complexes: Synthesis, self-coordinating aggregation properties, and catalytic studies. J. Porphyr. Phthalocyan 2002, 6, 50–57. [Google Scholar] [CrossRef]
- Tovmasyan, A.; Weitner, T.; Sheng, H.; Lu, M.; Rajic, Z.; Warner, D.S.; Spasojevic, I.; Reboucas, J.S.; Benov, L.; Batinic-Haberle, I. Differential coordination demands in Fe versus Mn water-soluble cationic metalloporphyrins translate into remarkably different aqueous redox chemistry and biology. Inorg. Chem. 2013, 52, 5677–5691. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, J.S.; Spasojević, I.; Batinić-Haberle, I. Quality of potent Mn porphyrin-based SOD mimics and peroxynitrite scavengers for pre-clinical mechanistic/therapeutic purposes. J. Pharm. Biomed. Anal. 2008, 48, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shi, L.; Li, Q.; Jiang, H.; Selke, M.; Ba, L.; Wang, X. Probing the dynamic effect of Cys-CdTe quantum dots toward cancer cells in vitro. Chem. Res. Toxicol. 2009, 23, 82–88. [Google Scholar] [CrossRef]
- Jett, B.; Hatter, K.; Huycke, M.; Gilmore, M. Simplified agar plate method for quantifying viable bacteria. Biotechniques 1997, 23, 648–650. [Google Scholar] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, O.S.; Ribeiro, M.S.; Rodas, A.C.D.; Rebouças, J.S.; Fontes, A.; Santos, B.S. Comparative Study on the Efficiency of the Photodynamic Inactivation of Candida albicans Using CdTe Quantum Dots, Zn(II) Porphyrin and Their Conjugates as Photosensitizers. Molecules 2015, 20, 8893-8912. https://doi.org/10.3390/molecules20058893
Viana OS, Ribeiro MS, Rodas ACD, Rebouças JS, Fontes A, Santos BS. Comparative Study on the Efficiency of the Photodynamic Inactivation of Candida albicans Using CdTe Quantum Dots, Zn(II) Porphyrin and Their Conjugates as Photosensitizers. Molecules. 2015; 20(5):8893-8912. https://doi.org/10.3390/molecules20058893
Chicago/Turabian StyleViana, Osnir S., Martha S. Ribeiro, Andréa C. D. Rodas, Júlio S. Rebouças, Adriana Fontes, and Beate S. Santos. 2015. "Comparative Study on the Efficiency of the Photodynamic Inactivation of Candida albicans Using CdTe Quantum Dots, Zn(II) Porphyrin and Their Conjugates as Photosensitizers" Molecules 20, no. 5: 8893-8912. https://doi.org/10.3390/molecules20058893
APA StyleViana, O. S., Ribeiro, M. S., Rodas, A. C. D., Rebouças, J. S., Fontes, A., & Santos, B. S. (2015). Comparative Study on the Efficiency of the Photodynamic Inactivation of Candida albicans Using CdTe Quantum Dots, Zn(II) Porphyrin and Their Conjugates as Photosensitizers. Molecules, 20(5), 8893-8912. https://doi.org/10.3390/molecules20058893