Synthesis and Biological Evaluation of 2-Picolylamide-Based Diselenides with Non-Bonded Interactions
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry


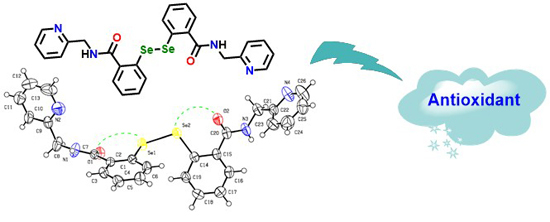
2.2. X-ray Crystallography

2.3. Biological Evaluation
2.3.1. Thiol Peroxidase-Like (TPx) Activity
| Compound | Concentration (µM) | |||
|---|---|---|---|---|
| 15 | 25 | 75 | 100 | |
| 5a | 1.16 | 3.88 | 27.60 | 34.00 |
| 5b | 0.01 | 0.01 | 1.72 | 3.40 |
| 5c | 9.71 | 14.97 | 24.03 | 31.91 |
| 5d | 20.74 | 31.34 | 41.66 | 46.48 |
| DPDS | 9.96 | |||
2.3.2. Inhibition of Thiobarbituric Acid Reactive Substances (TBARS) Production in Brain Homogenates

3. Experimental Section
3.1. General Methods and Materials
3.1.1. General Procedure for the Synthesis of Diselenide 7
3.1.2. Procedure for the Synthesis of Diselenide 9
3.1.3. General Procedure for the Synthesis of 2-Picolyamide Derivatives of Diselenides 5a–d
3.2. General Methods and Materials
3.2.1. Glutathione-Peroxidase-Like Activity Assay
3.2.2. Animals
3.2.3. Thiobarbituric Acid Reactive Substances (TBARS) Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Back, T.G. Design and synthesis of some biologically interesting natural and unnatural products based on organosulfur and selenium chemistry. Can. J. Chem. 2009, 87, 1657–1674. [Google Scholar] [CrossRef]
- Saba, S.; Rafique, J.; Braga, A.L. Synthesis of unsymmetrical diorganyl chalcogenides under greener conditions: Use of an iodine/DMSO system, solvent- and metal-free approach. Adv. Synth. Catal. 2015, 357, 1446–1452. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.-W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Mugesh, G. Functional mimics of glutathione peroxidase: Bioinspired synthetic antioxidants. Acc. Chem. Res. 2010, 43, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Rappoport, Z. The Chemistry of Organic Selenium and Tellurium Compounds; Wiley & Sons, Ltd.: Chichester, UK, 2014; Volume 4. [Google Scholar]
- Devillanova, F.A.; du Mont, W.-W. Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, 2nd ed.; RSC: Cambridge, UK, 2013. [Google Scholar]
- Back, T.G. Organoselenium Chemistry—A Practical Approach, 1st ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Godoi, M.; Paixão, M.W.; Braga, A.L. Chiral organoselenium-transition-metal catalysts in asymmetric transformations. Dalton Trans. 2011, 40, 11347–11355. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.V.; Wirth, T. Facile oxidative rearrangements using hypervalent iodine reagents. Org. Lett. 2011, 13, 6504–6507. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, Y.J.; Lee, H.; Park, K.Y.; Lee, C.; Chin, C.S. Ionic liquids containing anionic selenium species: Applications for the oxidative carbonylation of aniline. Angew. Chem. Int. Ed. 2002, 41, 4300–4303. [Google Scholar] [CrossRef]
- Nicolaour, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part A. Antioxidant compounds. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; Wiley: Chichester, UK, 2013; Volume 4, pp. 989–1052. [Google Scholar]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part B. Anti-infective and anticancer Compounds. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; Wiley: Chichester, UK, 2013; Volume 4, pp. 1053–1117. [Google Scholar]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part C. Miscellaneous biological activities. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; Wiley: Chichester, UK, 2013; Volume 4, pp. 1119–1174. [Google Scholar]
- Frizon, T.E.; Rafique, J.; Saba, S.; Bechtold, I.H.; Gallardo, H.; Braga, A.L. Synthesis of functionalized organoselenium materials: Selenides and diselenides containing cholesterol. Eur. J. Org. Chem. 2015, 3470–3476. [Google Scholar] [CrossRef]
- Shamberger, R.J. Biochemistry of Selenium; Plenum Press: New York, NY, USA, 1983. [Google Scholar]
- Wessjohann, L.A.; Schneider, A.; Abbas, M.; Brandt, W. Selenium in chemistry and biochemistry in comparison to sulfur. Biol. Chem. 2007, 388, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealised. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Tiecco, M.; Testaferri, L.; Santi, C.; Tomassini, C.; Santoro, S.; Marini, F.; Bagnoli, L.; Temperini, A. Intramolecular non-bonding interaction between selenium and sulfur. Spectroscopic evidences and importance in asymmetric synthesis. Eur. J. Org. Chem. 2006, 4867–4873. [Google Scholar] [CrossRef]
- Bleiholder, C.; Werz, D.B.; Koppel, H.; Gleiter, R. Theoretical investigations on chalcogen-chalcogen interactions: What makes these nonbonded interactions bonding? J. Am. Chem. Soc. 2006, 128, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Baca, I.; Chivers, T. Weakly bonding interactions in organochalcogen chemistry. Phorphorus Sulfur Silicon Relat. Elem. 2000, 164, 207–227. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Itoh, N. First linear alignment of five C–Se…O…Se–C atoms in anthraquinone and 9-(methoxy)anthracene bearing phenylselanyl groups at 1,8-positions. Chem. Commun. 2003, 124–125. [Google Scholar] [CrossRef]
- Mugesh, G.; Panda, A.; Singh, H.B.; Butcher, R.J. Intramolecular Se…N nonbonding interactions in low-valent organoselenium derivatives: A detailed study by 1H and 77Se NMR spectroscopy and X-Ray crystallography. Chem. Eur. J. 1999, 5, 1411–1421. [Google Scholar]
- Iwaoka, M.; Tomoda, S. Nature of the Intramolecular Se…N nonbonded interaction of 2-selenobenzylamine derivatives. An experim,ental evaluation by 1H, 77Se, and 15N-NMR spectroscopy. J. Am. Chem. Soc. 1996, 118, 8077–8084. [Google Scholar] [CrossRef]
- Iwaoka, M.; Komatsu, H.; Katsuda, T.; Tomoda, S. Experimental and theoretical studies on the nature of weak nonbonded interactions between divalent selenium and halogen atoms. J. Org. Chem. 2005, 70, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Minkin, V.I.; Minyaev, R.M. Cyclic aromatic systems with hypervalent centers. Chem. Rev. 2001, 101, 1247–1265. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Patel, C.; Liebman, J.F.; Sunoj, R.B. Probing intramolecular interactions in arylselenides using a property descriptor based approach. J. Phys. Chem. A 2008, 112, 8797–8803. [Google Scholar] [CrossRef] [PubMed]
- Press, D.J.; Mercier, E.A.; Kuzma, D.; Back, T.G. Substituent effects upon the catalytic activity of aromatic cyclic seleninate esters and spirodioxyselenuranes that act as glutathione peroxidase mimetics. J. Org. Chem. 2008, 73, 4252–4255. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Mugesh, G. A simple and efficient strategy to enhance the antioxidant activities of amino-substituted glutathione peroxidase mimics. Chem. Eur. J. 2008, 14, 8640–8651. [Google Scholar] [CrossRef] [PubMed]
- Nasimento, V.; Ferreira, N.L.; Canto, R.F.S.; Schott, K.L.; Waczuk, E.P.; Sancineto, L.; Santi, C.; Rocha, J.B.T.; Braga, A.L. Synthesis and biological evaluation of new nitrogen-containing diselenides. Eur. J. Med. Chem. 2014, 87, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, V.; Alberto, E.E.; Tondo, D.W.; Dambrowski, D.; Detty, M.R.; Nome, F.; Braga, A.L. GPx-like activity of selenides and selenoxides: Experimental evidence for the involvement of hydroxy perhydroxy selenane as the active species. J. Am. Chem. Soc. 2012, 134, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Narayanaperumal, S.; Rahman, A.U.; Braga, A.L.; Rodrigues, O.E.D.; Rocah, J.B.T.; Gul, K. Modulation of diorganoyl dichalcogenides reactivity by non-bonded nitrogen interactions. Chem. Biol. Interact. 2012, 199, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Hassan, W.; Anwar, J.; Deobald, A.M.; Kamdem, J.P.; SOUZA, D.O.; Rocha, J.B.T. 1-(2-(2-(2-(1-aminoethyl) phenyl)diselanyl)phenyl)ethanamine: An amino Organoselenium compound with interesting antioxidant profile. Toxicol. In Vitro 2014, 28, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Schair, V.P.P.; Santos, D.B.; Duarte, M.M.M.F.; Varga, F.; Nogueira, C.W.; Zeni, G.; Braga, A.L.; Rocha, J.B.T. Addition of butoxycarbonyl group to phenylalanine derived chalcogenide increases the toxic potential: Importance of non-bonding nitrogen interaction. Chem. Biol. Interact. 2014, 207, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Pingawer, R.; Tongraung, P.; Woracharteewan, A.; Nantasenamet, C.; Prahayasittikul, S.; Ruchiarwat, S.; Prachayasittikul, V. Cytotoxicity and QSAR study of (thio)ureas derived from phenylalkylamines and pyridylalkylamines. Med. Chem. Res. 2013, 22, 4016–4029. [Google Scholar]
- Xue, L.; Wang, H.-H.; Wang, X.-J.; Jiang, H. Modulating affinities of di-2-picolylamine (dpa)-substituted quinoline sensors for zinc ions by varying pendant ligands. Inorg. Chem. 2008, 47, 4310–4318. [Google Scholar] [CrossRef] [PubMed]
- Pullimamidi, R.R.; Nomula, R.; Pallepogu, R.; Shaik, H. Picolinic acid based Cu(II) complexes with heterocyclic bases—Crystal structure, DNA binding and cleavage studies. Eur. J. Med. Chem. 2014, 79, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-K.; Law, W.H.T.; Liu, H.-W.; Lo, K.K.-W. Luminescent cyclometalated iridium(iii) polypyridine di-2-picolylamine complexes: Synthesis, photophysics, electrochemistry, cation binding, cellular internalization, and cytotoxic activity. Inorg. Chem. 2011, 50, 8570–8579. [Google Scholar] [CrossRef] [PubMed]
- Krief, A.; Derock, M. Condition-driven selective syntheses of dialkyl diselenides involving elemental selenium and sodium borohydride. Synlett 2005, 6, 1012–10140. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. Diselenides and allyl selenides as glutathione peroxidase mimetics. Remarkable activity of cyclic seleninates produced in situ by the oxidation of allyl ω-hydroxyalkyl selenides. J. Am. Chem. Soc. 2003, 125, 13455–13460. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Sheng, J.; Sun, Y.; Yang, S.; Lu, C.; Yan, J.; Liu, A.; Luo, H.-B.; Huang, L.; Li, X. Synthesis and evaluation of multi-target-directed ligands against alzheimer’s disease based on the fusion of donepezil and ebselen. J. Med. Chem. 2013, 56, 9089–9099. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, K.; Shah, P.; Singh, H.B.; Butcher, R.J. Synthesis, structure, and glutathione peroxidase-like activity of amino acid containing ebselen analogues and diaryl diselenides. Chem. Eur. J. 2011, 17, 12741–12755. [Google Scholar] [CrossRef] [PubMed]
- Sarma, B.K.; Mugesh, G. Antioxidant activity of the anti-inflammatory compound ebselen: A reversible cyclization pathway via selenenic and seleninic acid intermediates. Chem. Eur. J. 2008, 14, 10603–10614. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Nishibayashi, Y.; Uemura, S. Asymmetric baeyer-villiger oxidation of cyclic ketones using chiral organoselenium catalysts. Bull. Chem. Soc. Jpn. 2002, 75, 2233–2237. [Google Scholar] [CrossRef]
- Mugesh, G.; Panda, A.; Singh, H.B.; Punekar, N.S.; Butcher, R.J. Glutathione peroxidase-like antioxidant activity of diaryl diselenides- a mechanistic study. J. Am. Chem. Soc. 2001, 123, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Mugesh, G. Amide-based glutathione peroxidase mimics: Effect of secondary and tertiary amide substituents on antioxidant activity. Chem. Asian J. 2009, 4, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Yla-Herttuala, S. Oxidized LDL and atherogenesis. Ann. N. Y. Acad. Sci. 1999, 874, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Epp, O.; Ladenstein, R.; Wendel, A. The refined structure of the selenoenzyme glutathione peroxidase at 0.2 nm resolution. Eur. J. Biochem. 1983, 133, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.S.; Prestes, A.S.; Wagner, C.; Sudati, J.H.; Alves, D.; Porciúncula, L.O.; Kade, I.J.; Rocha, J.B.T. Reduction of diphenyl diselenide and analogs by mammalian thioredoxin reductase is independent of their gluthathione peroxidase-like activity: A possible novel pathway for their antioxidant activity. Molecules 2010, 15, 7699–7714. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K. Diamond 3.1a. 1997–2005, Version 1.1a; Crystal Impact GbR: Bonn, Germany, 2005.
- Kurz, J.L.; Harris, J.C. Substituent effect transmission from heavy atoms. Microscopic dissociation constants of selenoglycolic acid. J. Org. Chem. 1970, 35, 3086–3090. [Google Scholar] [CrossRef]
- Painter, E.P.; Franke, K.W.; Gortner, R.A. Organic selenium compounds. Their decomposition in alkaline solutions, and other properties related to the behavior of selenium compounds in cereals. J. Org. Chem. 1940, 5, 579–589. [Google Scholar] [CrossRef]
- Günther, W.H.H. Hypophosphorous acid, a novel reagent for the reduction of diselenides and the selenol-catalyzed reduction of disulfides. J. Org. Chem. 1966, 31, 1202–1205. [Google Scholar] [CrossRef]
- Iwaoka, M.; Tomoda, S. A model study on the effect of an amino group on the antioxidant activity of glutathione peroxidase. J. Am. Chem. Soc. 1994, 116, 2557–2561. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 5a–d are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafique, J.; Saba, S.; Canto, R.F.S.; Frizon, T.E.A.; Hassan, W.; Waczuk, E.P.; Jan, M.; Back, D.F.; Da Rocha, J.B.T.; Braga, A.L. Synthesis and Biological Evaluation of 2-Picolylamide-Based Diselenides with Non-Bonded Interactions. Molecules 2015, 20, 10095-10109. https://doi.org/10.3390/molecules200610095
Rafique J, Saba S, Canto RFS, Frizon TEA, Hassan W, Waczuk EP, Jan M, Back DF, Da Rocha JBT, Braga AL. Synthesis and Biological Evaluation of 2-Picolylamide-Based Diselenides with Non-Bonded Interactions. Molecules. 2015; 20(6):10095-10109. https://doi.org/10.3390/molecules200610095
Chicago/Turabian StyleRafique, Jamal, Sumbal Saba, Rômulo Faria Santos Canto, Tiago Elias Allievi Frizon, Waseem Hassan, Emily Pansera Waczuk, Maryam Jan, Davi Fernando Back, João Batista Teixeira Da Rocha, and Antonio Luiz Braga. 2015. "Synthesis and Biological Evaluation of 2-Picolylamide-Based Diselenides with Non-Bonded Interactions" Molecules 20, no. 6: 10095-10109. https://doi.org/10.3390/molecules200610095
APA StyleRafique, J., Saba, S., Canto, R. F. S., Frizon, T. E. A., Hassan, W., Waczuk, E. P., Jan, M., Back, D. F., Da Rocha, J. B. T., & Braga, A. L. (2015). Synthesis and Biological Evaluation of 2-Picolylamide-Based Diselenides with Non-Bonded Interactions. Molecules, 20(6), 10095-10109. https://doi.org/10.3390/molecules200610095