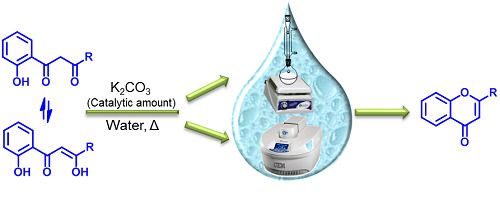
Synthesis of (E)-2-Styrylchromones and Flavones by Base-Catalyzed Cyclodehydration of the Appropriate β-Diketones Using Water as Solvent
Abstract
:1. Introduction



2. Results and Discussion

| Entry | Compound | Base/molar equiv | Time (h) | Yield of 5 (%) a |
|---|---|---|---|---|
| 1 | 4a R = H | K2CO3/1 | 4 b | 58 |
| 2 | 4a R = H | K2CO3/0.5 | 4 | 59 |
| 3 | 4a R = H | K2CO3/0.5 + TBAB/0.5 | 3 | 12 |
| 4 | 4a R = H | TMAOH/1 | 2 | 19 |
| 5 | 4a R = H | TMAOH/0.5 | 2 | 47 |
| 6 | 4b R = OCH3 | K2CO3/0.5 | 4 | 61 |
| 7 | 4c R = Cl | K2CO3/0.5 | 4 | 70 |
| 8 | 4d R = CH3 | K2CO3/0.5 | 4 b | 70 |
| 9 | 4e R = NO2 | K2CO3/0.5 | 4 | 20 |
| 10 | 4a R = H | K2CO3/0.5 | 4 | 55 c |
| Entry | Compound | Method | Base/molar equiv | Time (min) | Yield of 5 (%) a |
|---|---|---|---|---|---|
| 1 | 4a R =H | Open | K2CO3/1 | 10 | 35 |
| 2 | 4a R =H | Open | K2CO3/0.5 | 5 | 54 |
| 3 | 4a R = H | Open | K2CO3/0.5 | 30 | 66 |
| 4 | 4b R = OCH3 | Open | K2CO3/0.5 | 10 | 47 |
| 5 | 4b R = OCH3 | Open | K2CO3/0.5 | 30 | 56 |
| 6 | 4c R = Cl | Open | K2CO3/0.5 | 10 | 58 |
| 7 | 4c R = Cl | Open | K2CO3/0.5 | 30 | 47 |
| 8 | 4d R = CH3 | Open | K2CO3/0.5 | 10 | 45 |
| 9 | 4d R = CH3 | Open | K2CO3/0.5 | 30 | 55 |
| 10 | 4e R = NO2 | Open | K2CO3/0.5 | 10 | 56 |
| 11 | 4e R = NO2 | Open | K2CO3/0.5 | 30 | 38 |
| 12 | 4a R =H | Closed | K2CO3/0.5 | 5 | 50 |
| 13 | 4a R = H | Closed | K2CO3/0.5 | 30 | 67 |
| 14 | 4b R = OCH3 | Closed | K2CO3/0.5 | 30 | 77 |
| 15 | 4c R = Cl | Closed | K2CO3/0.5 | 10 | 66 |
| 16 | 4c R = Cl | Closed | K2CO3/0.5 | 15 | 38 |
| 17 | 4c R = Cl | Closed | K2CO3/0.5 | 30 | 41 |
| 18 | 4d R = CH3 | Closed | K2CO3/0.5 | 30 | 57 |
| 19 | 4e R = NO2 | Closed | K2CO3/0.5 | 30 | 27 |
| 20 | 4e R = NO2 | Closed | TMAOH/0.5 | 10 | 60 |
| Entry | Compound | Base/molar equiv | Time (min) | Yield of 5 (%) a |
|---|---|---|---|---|
| 1 | 4a R = H | K2CO3/0.05 | 30 | 65 |
| 2 | 4b R = OCH3 | K2CO3/0.05 | 30 | 75 |
| 3 | 4c R = Cl | K2CO3/0.05 | 30 | 64 |
| 4 | 4d R = CH3 | K2CO3/0.05 | 30 | 68 |
| 5 | 4e R = NO2 | K2CO3/0.05 | 30 | 69 |
| Entry | Compound | Method | Reaction Time (min) | Yield of 7 (%) a |
|---|---|---|---|---|
| 1 | 6a R1 =CH3, R2 = H | Oil bath | 60 | Quantitative |
| 2 | 6a R1 =CH3, R2 = H | Microwave | 15 | 70 |
| 3 | 6a R1 =CH3, R2 = H | Microwave | 30 | Quantitative |
| 4 | 6b R1 =H, R2 = OBn | Microwave | 30 | 45 |
| 5 | 6b R1 =H, R2 = OBn | Microwave | 45 | 46 |

3. Experimental Section
3.1. General Information
3.2. Optimized Experimental Procedure for the Cyclodehydration Reaction of β-Diketones 4a–e and 6a under Classical Heating Conditions
3.3. Optimized Experimental Procedure for the Cyclodehydration Reaction of β-Diketones 4a–e under Microwave Irradiation in Open Vessels
3.4. Optimized Experimental Procedure for the Cyclodehydration Reaction of β-Diketones 4a–e and 6a,b under Microwave Irradiation in Closed Vessels
| Compounds | R | Yield | Melting Point (°C) | Melting Point (°C) (Lit.) [50,51] |
|---|---|---|---|---|
| 5a | H | 67 | 136–137 | 133–134 |
| 5b | OCH3 | 77 | 125–126 | 139–140 a |
| 5c | Cl | 70 | 218–219 | 224–226 |
| 5d | CH3 | 70 | 158–159 | 159–160 |
| 5e | NO2 | 60 | 276–278 | 282–283 |
| Compounds | R1 | R2 | Yield | Melting Point (°C) | Melting Point (°C) (Lit.) |
|---|---|---|---|---|---|
| 7a | CH3 | H | Quant. | 73–74 | 110–112 a [52] |
| 7b | H | OBn | 46 | 82–84 | 82–83 [53] |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cook, C.; Samman, S. Flavonoids-chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Alam, S. Synthesis, antibacterial and antifungal activity of some derivatives of 2-phenyl-chromen-4-one. J. Chem. Sci. 2004, 116, 325–331. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Yi, Y.; Lee, K. Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorg. Med. Chem. Lett. 2003, 13, 1813–1815. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, H.J.; Ryu, J.-H. In vitro anti-inflammatory activity of 3-O-methyl-flavones isolated from Siegesbeckia glabrescens. Bioorg. Med. Chem. Lett. 2008, 18, 1511–1514. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Ozawa, K.; Ohtani, K.; Kasai, R.; Yamasaki, K. Antihistaminic flavones and aliphatic glycosides from Mentha spicata. Phytochemistry 1998, 48, 131–136. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Lu, B.-N.; Peng, J.-Y. Hepatoprotective activity of the total flavonoids from Rosa laevigata Michx fruit in mice treated by paracetamol. Food Chem. 2011, 125, 719–725. [Google Scholar] [CrossRef]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, M.; Marder, M.; Blank, V.C.; Roguin, L.P. Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorg. Med. Chem. 2006, 14, 2966–2971. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Lopez, A.; van Duyne, G.D.; Clardy, J.; Ortiz, W.; Baez, A. Hormothamnione, a novel cytotoxic styrylchromone from the marine cyanophyte hormothamnion enteromorphoides grunow. Tetrahedron Lett. 1986, 27, 1979–1982. [Google Scholar] [CrossRef]
- Gerwick, W.H. 6-Desmethoxyhormothamnione, a new cytotoxic styrylchromone from the marine cryptophyte Chrysophaeum taylori. J. Nat. Prod. 1989, 52, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Lee, M.K.; Sung, S.H.; Kim, Y.C. Neuroprotective 2-(2-phenylethyl)chromones of Imperata cylindrical. J. Nat. Prod. 2006, 69, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qiao, L.; Xie, D.; Yuan, Y.; Chen, N.; Dai, J.; Guo, S. 2-(2-Phenylethyl)chromones from Chinese eaglewood. Phytochemistry 2012, 76, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Yang, Y.; Liu, J.-H. Platachromone A–D: Cytotoxic 2-styrylchromones from the bark of Platanus × acerifolia (Aiton) Willd. Phytochem. Lett. 2013, 6, 387–391. [Google Scholar] [CrossRef]
- Silva, A.M.S.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Lévai, A.; Patonay, T. Synthesis and reactivity of styrylchromones. Arkivoc 2004, vii, 106–123. [Google Scholar] [CrossRef]
- Gomes, A.; Freitas, M.; Fernandes, E.; Lima, J.L.F.C. Biological activity of 2-styrylchromones. Mini Rev. Med. Chem. 2010, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Neuwirth, O.; Freitas, M.; Couto, D.; Ribeiro, D.; Figueiredo, A.G.P.R.; Silva, A.M.S.; Seixas, R.S.G.R.; Pinto, D.C.G.A.; Tomé, A.C.; et al. Synthesis and antioxidant properties of new chromone derivatives. Bioorg. Med. Chem. 2009, 17, 7218–7226. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.Y.; Chang, C.Y.; Liau, H.H.; Lu, P.J.; Chen, H.L.; Yang, C.N.; Li, H.Y. Synthesis of 2-styrylchromones as a novel class of antiproliferative agents targeting carcinoma cells. Eur. J. Med. Chem. 2009, 44, 2552–2562. [Google Scholar] [CrossRef] [PubMed]
- Doria, G.; Romeo, C.; Forgione, A.; Sberze, P.; Tibolla, N.; Corno, M.L.; Cruzzola, G.; Cadelli, G. Antiallergic agents. III. Substituted trans-2-ethenyl-4-oxo-4H-1-benzopyran-6-carboxylic acids. Eur. J. Med. Chem. 1979, 14, 347–351. [Google Scholar]
- Conti, C.; Mastromarino, P.; Goldoni, P.; Portalone, G.; Desideri, N. Synthesis and anti-rhinovirus properties of fluoro-substituted flavonoids. Antivir. Chem. Chemother. 2005, 16, 267–276, and references cited therein. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.; Carvalho, F.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Bastos, M.L. 2-Styrylchromones as novel inhibitors of xanthine oxidase. A structure-activity study. J. Enzym. Inhib. Med. Chem. 2002, 17, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.; Carvalho, M.; Carvalho, F.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Bastos, M.L. Hepatoprotective activity of polyhydroxylated 2-stryrylchromones against tert-butylhydroperoxide induced toxicity in freshly isolated rat hepatocytes. Arch. Toxicol. 2003, 77, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Filipe, P.; Silva, A.M.S.; Morliere, P.; Brito, C.M.; Patterson, L.K.; Hug, G.L.; Silva, J.N.; Cavaleiro, J.A.S.; Maziere, J.C.; Freitas, J.P.; et al. Polyhydroxylated 2-styrylchromones as potent antioxidants. Biochem. Pharmacol. 2004, 67, 2207–2218, and references cited therein. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.C.G.A.; Silva, A.M.S.; Cavaleiro, J.A.S. A convenient synthesis of new (E)-5-hydroxy-2-styrylchromones by modifications of the Baker-Venkataraman method. New J. Chem. 2000, 24, 85–92. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, S.; Makrandi, J.K. Solid phase Baker-Venkataraman rearrangement under solvent-free condition using grinding technique. Green Chem. Lett. Rev. 2009, 2, 53–55. [Google Scholar] [CrossRef]
- Baker, W. Molecular rearrangement of some o-acyloxyacetophenones and the mechanism of the production of 3-acylchromones. J. Chem. Soc. 1933, 1381–1389. [Google Scholar] [CrossRef]
- Mahal, H.S.; Venkataraman, K. Synthetical experiments in the chromone group. Part XIV. The action of sodamide on 1-acyloxy-2-acetonaphthones. J. Chem. Soc. 1934, 1767–1769. [Google Scholar] [CrossRef]
- Mughal, E.U.; Ayaz, M.; Hussain, Z.; Hasan, A.; Sadiq, A.; Riaz, M.; Malik, A.; Hussain, S.; Choudhary, M.I. Synthesis and antibacterial activity of substituted flavones, 4-thioflavones and 4-iminoflavones. Bioorg. Med. Chem. 2006, 14, 4704–4711, and references cited therein. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T. Flavone. Org. Synth. 1952, 32, 72–76. [Google Scholar]
- Hoshino, Y.; Takeno, N. A facile preparation of flavones using non aqueous cation-exchange resin. Bull. Chem. Soc. Jpn. 1987, 60, 1919–1920. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Mereddy, A.R. Microwave-assisted synthesis of functionalized flavones and chromones. Tetrahedron Lett. 2005, 46, 6315–6317. [Google Scholar] [CrossRef]
- Sarda, S.; Pathan, M.; Paike, V.; Pachmase, P.; Jadhav, W.; Pawar, R. A facile synthesis of flavones using recyclable ionic liquid under microwave irradiation. ARKIVOC 2006, xvi, 43–48. [Google Scholar] [CrossRef]
- Bennardi, D.O.; Romanelli, G.P.; Jios, J.L.; Autino, J.C.; Baronetti, G.T.; Thomas, H.J. Synthesis of substituted flavones and chromones using a Wells-Dawson heteropolyacid as catalyst. ARKIVOC 2008, xi, 123–130. [Google Scholar] [CrossRef]
- Bennardi, D.O.; Ruiz, D.M.; Romanelli, G.P.; Baronetti, G.T.; Thomas, H.J.; Autino, J.C. Efficient microwave solvent-free synthesis of flavones, chromones, coumarins and dihydrocoumarins. Lett. Org. Chem. 2009, 5, 607–615. [Google Scholar] [CrossRef]
- Romanelli, G.; Virla, E.; Duchowicz, P.; Gaddi, A.; Ruiz, D.; Bennardi, D.; Dell Valle Ortiz, D.; Autino, J. Sustainable synthesis of flavonoid derivatives, QSAR study and insecticidal activity against the fall armyworm, Spodoptera frugiperda (Lep.: Noctuidae). J. Agric. Food Chem. 2010, 8, 6290–6295. [Google Scholar] [CrossRef] [PubMed]
- Bennardi, D.O.; Romanelli, G.P.; Autino, J.C.; Pizzio, L.R. Trifluoromethanesulfonic acid supported on carbon used as catalysts in the synthesis of flavones and chromones. Catal. Commun. 2009, 10, 576–581. [Google Scholar] [CrossRef]
- Sharma, D.; Makrandi, J.K. A green synthesis of 2-phenyl/2-styrylchromones under solvent-free conditions using grinding technique. Green Chem. Lett. Rev. 2009, 2, 157–159. [Google Scholar] [CrossRef]
- Allen, M.; Evans, D.F.; Lumry, R. Thermodynamic Properties of the Ethylammonium Nitrate + Water System: Partial Molar Volumes, Heat Capacities, and Expansivities. J. Solut. Chem. 1985, 14, 549–560. [Google Scholar] [CrossRef]
- Aridoss, G.; Laali, K.K. Building Heterocyclic Systems with RC(OR)2+ Carbocations in Recyclable Brønsted Acidic Ionic Liquids: Facile Synthesis of 1-Substituted 1H-1,2,3,4-Tetrazoles, Benzazoles and Other Ring Systems with CH(OEt)3 and EtC(OEt)3 in [EtNH3][NO3] and [PMIM(SO3H)][OTf]. Eur. J. Org. Chem. 2011, 2011, 2827–2835. [Google Scholar]
- Seijas, J.A.; Vázquez-Tato, M.P.; Carballido-Reboredo, R. Solvent-Free Synthesis of Functionalized Flavones under Microwave Irradiation. J. Org. Chem. 2005, 70, 2855–2858. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R. Hydrophobic effects on simple organic reactions in water. Acc. Chem. Res. 1991, 24, 159–164. [Google Scholar] [CrossRef]
- Li, C.-J. Organic reactions in aqueous media with a focus on carbon-carbon bond formations: A decade update. Chem. Rev. 2005, 105, 3095–3165. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.T. Solvents from nature. Green Chem. 2008, 10, 1024–1028. [Google Scholar] [CrossRef]
- Hauser, C.R.; Swamer, F.W.; Ringler, B.I. Alkaline cleavage of unsymmetrical β-diketones. Ring opening of acylcylohexanones to form ε-acyl caproic acids. J. Am. Chem. Soc. 1948, 70, 4023–4026. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.M.; Silva, A.M.S.; Cavaleiro, J.A.S. Synthesis of new hydroxy-2-styrylchromones. Eur. J. Org. Chem. 2003, 4575–4585. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Microwave-assisted synthesis in water as solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef] [PubMed]
- Chee, C.F.; Buckle, M.J.C.; Rahman, N.A. An efficient one-pot synthesis of flavones. Tetrahedron Lett. 2011, 52, 3120–3123. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Fu, H. K2CO3-Catalyzed synthesis of chromones and 4-quinolones through the cleavage of aromatic C-O bonds. Org. Lett. 2012, 14, 2710–2713. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.C.G.A.; Silva, A.M.S.; Almeida, L.M.P.M.; Cavaleiro, J.A.S.; Lévai, A.; Patonay, T. Synthesis of 4-aryl-3-(2-chromonyl)-2-pyrazolines by the 1,3-dipolar cycloaddition of 2-styrylchromones with diazomethane. J. Heterocycl. Chem. 1998, 35, 217–224. [Google Scholar] [CrossRef]
- Barros, A.I.R.N.A.; Silva, A.M.S. Synthesis and structure elucidation of three series of nitro-2-styrylchromones using 1D and 2D NMR spectroscopy. Magn. Reson. Chem. 2009, 47, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, B.S.; Alam, A.; Nayak, Y.; Kumar, D.V. Synthesis of 3-methylflavones and their antioxidant and antibacterial activities. Med. Chem. Res. 2012, 21, 1991–1996. [Google Scholar] [CrossRef]
- Riva, C.; De Toma, C.; Donadel, L.; Boi, C.; Pennini, R.; Motta, G.; Leonardi, A. New DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) assisted one-pot synthesis of 2,8-disubstituted 4H-1-benzopyran-4-ones. Synthesis 1997, 195–201. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4a–e, 5a–e, 6a,b and 7a,b are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, J.; Silva, V.L.M.; Silva, A.M.G.; Silva, A.M.S. Synthesis of (E)-2-Styrylchromones and Flavones by Base-Catalyzed Cyclodehydration of the Appropriate β-Diketones Using Water as Solvent. Molecules 2015, 20, 11418-11431. https://doi.org/10.3390/molecules200611418
Pinto J, Silva VLM, Silva AMG, Silva AMS. Synthesis of (E)-2-Styrylchromones and Flavones by Base-Catalyzed Cyclodehydration of the Appropriate β-Diketones Using Water as Solvent. Molecules. 2015; 20(6):11418-11431. https://doi.org/10.3390/molecules200611418
Chicago/Turabian StylePinto, Joana, Vera L. M. Silva, Ana M. G. Silva, and Artur M. S. Silva. 2015. "Synthesis of (E)-2-Styrylchromones and Flavones by Base-Catalyzed Cyclodehydration of the Appropriate β-Diketones Using Water as Solvent" Molecules 20, no. 6: 11418-11431. https://doi.org/10.3390/molecules200611418
APA StylePinto, J., Silva, V. L. M., Silva, A. M. G., & Silva, A. M. S. (2015). Synthesis of (E)-2-Styrylchromones and Flavones by Base-Catalyzed Cyclodehydration of the Appropriate β-Diketones Using Water as Solvent. Molecules, 20(6), 11418-11431. https://doi.org/10.3390/molecules200611418