Synthesis of Radiation Curable Palm Oil–Based Epoxy Acrylate: NMR and FTIR Spectroscopic Investigations
Abstract
:1. Introduction
2. Results and Discussion
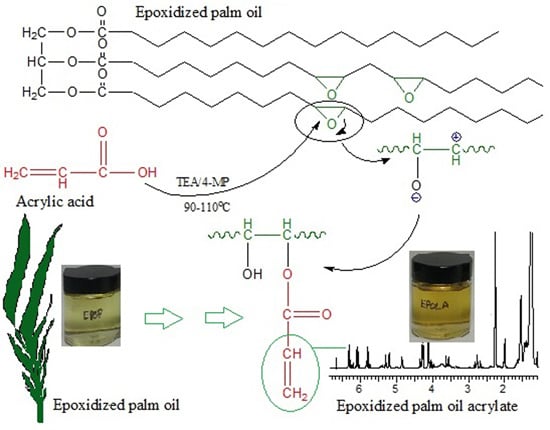
2.1. Epoxidized Palm Oil Acrylate (EPOLA)


| Resin | Acid Value (mg KOH/g) | Iodine Value (I2/100g) | OH-Value (mg KOH/g) | Ox-O2%/100 g | Viscosity (cPs) at 25 °C | Density (g/cm3) |
|---|---|---|---|---|---|---|
| EPOP | 2.5 | 0.76 | 12.9 | 3.0 | 280 | 0.9095 |
| EPOLA | 10.2 | 20.7 | 50.99 | 0.2 | 580 | 0.9748 |
| Purified EPOLA | 1.5 | 18.6 | 44.9 | --- | 1350 | 0.9941 |

| Resin | Mw (g/mol) | Mn (g/mol) | PDI (Mw/Mn) |
|---|---|---|---|
| EPOP | 1262 | 925 | 1.3 |
| EPOLA | 1667 | 1053 | 1.5 |

| Functional Group | FTIR Absorption (cm−1) | |
|---|---|---|
| EPOP | EPOLA | |
| OH stretching | 3472 | 3470 |
| C-H stretching (CH3) | 2924 | 2924 |
| C-H stretching (CH2) | 2853 | 2853 |
| C=O stretching | 1743 | 1744 |
| C=O (carboxylic group) | ----- | 1725 |
| CH2=CH-R stretching | ----- | 1636 |
| CH2=CH-R Scissoring | ----- | 1406 |
| C=O stretching | 1743 | 1744 |
| C=O (carboxylic group) | ----- | 1725 |
| CH2=CH-R stretching | ----- | 1636 |
| CH2=CH-R Scissoring | ----- | 1406 |
| C-O-C stretching | 1240 | ----- |
| C-O-C asymmetric bending | 835 | ----- |
| CH=C-H out of plane bending | ------ | 810 |
2.2. NMR Analysis of EPOP and EPOLA




2.3. Characterization of the Solid Cured Films of EPOLA




| EPOLA | T5%/°C | T10%/°C | T50%/°C | Tmax/°C | Tg/°C | Char (%) |
|---|---|---|---|---|---|---|
| Irg-184 | 308.5 | 359.2 | 440.3 | 506.0 | 119.6 | 2.34 |
| D-1173 | 277.0 | 354.0 | 444.0 | 496.0 | 115.5 | 2.30 |


| EPOLA | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| Irg-184 | 6.2(±0.7) | 65.7(±5.0) | 8.7(±0.15) |
| D-1173 | 5.2(±1.2) | 53.6(±2.3) | 12.5(±0.19) |
3. Experimental Section
3.1. Materials
3.2. Synthesis of Epoxidized Palm Oil Acrylate
3.3. Preparation of EPOlA Cured Films by UV Free Radical Polymerization
3.4. Characterization of Epoxidized Palm Oil Acrylate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seniha Güner, F.; Yağcı, Y.; Tuncer Erciyes, A. Polymers from triglyceride oils. Prog. Polym. Sci. 2006, 31, 633–670. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J.J. The use of renewable feedstock in UV-curable materials—A new age for polymers and green chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Petrović, Z.S. Polyurethanes from Vegetable Oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Childers, M.I.; Longo, J.M.; van Zee, N.J.; LaPointe, A.M.; Coates, G.W. Stereoselective epoxide polymerization and copolymerization. Chem. Rev. 2014, 114, 8129–8152. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Jiang, Y.; Liu, X.; Fan, L.; Zhu, J. Bio-based tetrafunctional crosslink agent from gallic acid and its enhanced soybean oil-based UV-cured coatings with high performance. RSC Adv. 2014, 4, 23036–23042. [Google Scholar] [CrossRef]
- Habib, F.; Bajpai, M. Synthesis and characterization of acrylated epoxidized soybean oil for UV cured coatings. Chem. Chem. Technol. 2011, 5, 317–326. [Google Scholar]
- Pelletier, H.; Gandini, A. Preparation of acrylated and urethanated triacylglycerols. Eur. J. Lipid Sci. Technol. 2006, 108, 411–420. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A. Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Kolot, V.; Grinberg, S. Vernonia oil–based acrylate and methacrylate polymers and interpenetrating polymer networks with epoxy resins. J. Appl. Polym. Sci. 2004, 91, 3835–3843. [Google Scholar] [CrossRef]
- Roose, P.; Fallais, I.; Vandermiers, C.; Olivier, M.G.; Poelman, M. Radiation curing technology: An attractive technology for metal coating. Prog. Org. Coat. 2009, 64, 163–170. [Google Scholar] [CrossRef]
- Masson, F.; Decker, C.; Jaworek, T.; Schwalm, R. UV-radiation curing of waterbased urethane–acrylate coatings. Prog. Org. Coat. 2000, 39, 115–126. [Google Scholar] [CrossRef]
- Mashouf, G.; Ebrahimi, M.; Bastani, S. UV curable urethane acrylate coatings formulation: Experimental design approach. Pigm. Resin Technol. 2014, 43, 61–68. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, D.S. Effect of clay content on the ultraviolet curing and physical properties of urethane acrylate/clay nanocomposites. Polym. Compos. 2007, 28, 325–330. [Google Scholar] [CrossRef]
- Keskin, S.; Usanmaz, A. Hydroxyl-terminated poly(urethane acrylate) as a soft liner in dental applications: Synthesis and characterization. J. Appl. Polym. Sci. 2010, 117, 458–466. [Google Scholar] [CrossRef]
- Santhosh, P.; Vasudevan, T.; Gopalan, A.; Lee, K.P. Preparation and properties of new cross-linked polyurethane acrylate electrolytes for lithium batteries. J. Power Sources 2006, 160, 609–620. [Google Scholar] [CrossRef]
- Ugur, M.; Kılıç, H.; Berkem, M.; Güngör, A. Synthesis by UV-curing and characterisation of polyurethane acrylate-lithium salts-based polymer electrolytes in lithium batteries. Chem. Pap. 2014, 68, 1561–1572. [Google Scholar] [CrossRef]
- Oprea, S.; Potolinca, V. The synthesis and properties of binary acrylate oligomer mixtures and their blends with different soybean oil contents. High Perform. Polym. 2013, 25, 822–831. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Shahi, V.K.; Schubert, R.; Rangarajan, R.; Mehnert, R. Development of urethane acrylate composite ion-exchange membranes and their electrochemical characterization. J. Colloid Interface Sci. 2004, 270, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Jang, E.S.; Song, J.H.; Choi, S.; Khan, S.B.; Han, H. Preparation and properties of poly(urethane acrylate) films for ultraviolet-curable coatings. J. Appl. Polym. Sci. 2010, 118, 2454–2460. [Google Scholar] [CrossRef]
- Džunuzović, E.S.; Tasić, S.V.; Božić, B.R.; Džunuzović, J.V.; Dunjić, B.M.; Jeremić, K.B. Mechanical and thermal properties of UV cured mixtures of linear and hyperbranched urethane acrylates. Prog. Org. Coat. 2012, 74, 158–164. [Google Scholar] [CrossRef]
- Degrandi-Contraires, E.; Lopez, A.; Reyes, Y.; Asua, J.M.; Creton, C. High-shear-strength waterborne polyurethane/acrylic soft adhesives. Macromol. Mater. Eng. 2012, 298, 612–623. [Google Scholar] [CrossRef]
- Tajau, R.; Mahmood, M.H.; Salleh, M.Z.; Mohd Dahlan, K.Z.; Che iSMaiL, R.; Muhammad Faisal, S.; Sheikh Abdul Rahman, S.M.Z.S. Production of UV-curable palm oil resins/oligomers using laboratory scale and pilot scale systems. Sains Malays. 2013, 42, 459–467. [Google Scholar]
- Cheong, M.Y.; Lye Ooi, T.; Ahmad, S.; Yunus, W.M.Z.W.; Kuang, D. Synthesis and characterization of palm-based resin for UV coating. J. Appl. Polym. Sci. 2009, 111, 2353–2361. [Google Scholar] [CrossRef]
- Salih, A.; Yunus, W.M.Z.W.; Dahlan, K.Z.M.; Mahmood, M.H.; Ahmad, M. UV-Curable Palm Oil Based-Urethane Acrylate/Clay Nanocomposites. Pertanika J. Sci. Technol. 2012, 20, 435–444. [Google Scholar]
- Sharma, B.; Adhvaryu, A.; Liu, Z.; Erhan, S. Chemical modification of vegetable oils for lubricant applications. J. Am. Oil Chem. Soc. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- Fu, L.; Yang, L.; Dai, C.; Zhao, C.; Ma, L. Thermal and mechanical properties of acrylated expoxidized-soybean oil-based thermosets. J. Appl. Polym. Sci. 2010, 117, 2220–2225. [Google Scholar] [CrossRef]
- Téllez, G.L.; Vigueras-Santiago, E.; Hernández-López, S. Characterization of linseed oil epoxidized at different percentages. Superf. Vacio 2009, 22, 5–10. [Google Scholar]
- Zhang, X.; Chen, L.; Zhang, Q.; Huang, Y.; Li, J. Synthesis and characterization of epoxidized acrylated natural rubber cross-linked by star-shaped polystyrene. Iran. Polym. J. 2011, 20, 55–63. [Google Scholar]
- Sharma, B.K.; Adhvaryu, A.; Erhan, S. Synthesis of hydroxy thio-ether derivatives of vegetable oil. J. Agric. Food Chem. 2006, 54, 9866–9872. [Google Scholar] [CrossRef] [PubMed]
- Adhvaryu, A.; Liu, Z.; Erhan, S.Z. Epoxidized soybean oil as a potential source of high-temperature lubricants. Ind. Crops Prod. 2002, 15, 247–254. [Google Scholar] [CrossRef]
- La Scala, J.; Wool, R.P. The effect of fatty acid composition on the acrylation kinetics of epoxidized triacylglycerols. J. Am. Oil Chem. Soc. 2002, 79, 59–63. [Google Scholar] [CrossRef]
- Lathi, P.S.; Mattiasson, B. Green approach for the preparation of biodegradable lubricant base stock from epoxidized vegetable oil. Appl. Catal. B 2007, 69, 207–212. [Google Scholar] [CrossRef]
- Adekunle, K.; Åkesson, D.; Skrifvars, M. Synthesis of reactive soybean oils for use as a biobased thermoset resins in structural natural fiber composites. J. Appl. Polym. Sci. 2010, 115, 3137–3145. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, F.L. Synthesis and characterization of UV-curable acrylic resin containing fluorine groups. Polym. Int. 2005, 54, 705–709. [Google Scholar] [CrossRef]
- Supanchaiyamat, N.; Hunt, A.J.; Shuttleworth, P.S.; Ding, C.; Clark, J.H.; Matharu, A.S. Bio-based thermoset composites from epoxidised linseed oil and expanded starch. RSC Adv. 2014, 4, 23304–23313. [Google Scholar] [CrossRef]
- Kardar, P.; Ebrahimi, M.; Bastani, S. Curing behaviour and mechanical properties of pigmented UV-curable epoxy acrylate coatings. Pigment Resin Technol. 2014, 43, 177–184. [Google Scholar] [CrossRef]
- Cui, J.; Yu, G.; Pan, C. A novel UV-curable epoxy acrylate resin containing arylene ether sulfone linkages: Preparation, characterization, and properties. J. Appl. Polym. Sci. 2014, 131, 41067–41075. [Google Scholar] [CrossRef]
- Yu, G.; Liu, C.; Li, X.; Wang, J.; Jian, X.; Pan, C. Highly thermostable rigid-rod networks constructed from an unsymmetrical bisphthalonitrile bearing phthalazinone moieties. Polym. Chem. 2012, 3, 1024–1032. [Google Scholar] [CrossRef]
- Li, Y.; Fu, L.; Lai, S.; Cai, X.; Yang, L. Synthesis and characterization of cast resin based on different saturation epoxidized soybean oil. Eur. J. Lipid Sci. Technol. 2010, 112, 511–516. [Google Scholar] [CrossRef]
- Gao, Q.; Li, H.; Zeng, X. UV-curing of hyperbranched polyurethane acrylate-polyurethane diacrylate/SiO2 dispersion and TGA/FTIR study of cured films. J. Cent. South Univ. Technol. 2012, 19, 63–70. [Google Scholar] [CrossRef]
- Sample Availability: Samples are not available from authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salih, A.M.; Ahmad, M.B.; Ibrahim, N.A.; Dahlan, K.Z.H.M.; Tajau, R.; Mahmood, M.H.; Yunus, W.M.Z.W. Synthesis of Radiation Curable Palm Oil–Based Epoxy Acrylate: NMR and FTIR Spectroscopic Investigations. Molecules 2015, 20, 14191-14211. https://doi.org/10.3390/molecules200814191
Salih AM, Ahmad MB, Ibrahim NA, Dahlan KZHM, Tajau R, Mahmood MH, Yunus WMZW. Synthesis of Radiation Curable Palm Oil–Based Epoxy Acrylate: NMR and FTIR Spectroscopic Investigations. Molecules. 2015; 20(8):14191-14211. https://doi.org/10.3390/molecules200814191
Chicago/Turabian StyleSalih, Ashraf M., Mansor Bin Ahmad, Nor Azowa Ibrahim, Khairul Zaman Hj Mohd Dahlan, Rida Tajau, Mohd Hilmi Mahmood, and Wan Md. Zin Wan Yunus. 2015. "Synthesis of Radiation Curable Palm Oil–Based Epoxy Acrylate: NMR and FTIR Spectroscopic Investigations" Molecules 20, no. 8: 14191-14211. https://doi.org/10.3390/molecules200814191
APA StyleSalih, A. M., Ahmad, M. B., Ibrahim, N. A., Dahlan, K. Z. H. M., Tajau, R., Mahmood, M. H., & Yunus, W. M. Z. W. (2015). Synthesis of Radiation Curable Palm Oil–Based Epoxy Acrylate: NMR and FTIR Spectroscopic Investigations. Molecules, 20(8), 14191-14211. https://doi.org/10.3390/molecules200814191