Synthesis, Antibacterial and Antitubercular Activities of Some 5H-Thiazolo[3,2-a]pyrimidin-5-ones and Sulfonic Acid Derivatives
Abstract
:1. Introduction

2. Results and Discussion
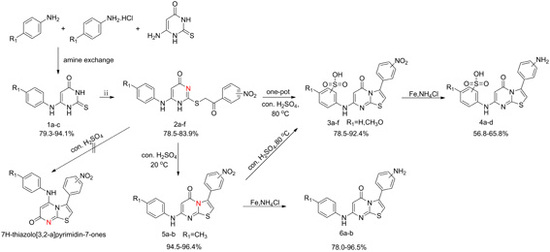
2.1. Chemistry



2.2. Biological Assays
| Compd. No. | Antibacterial Activity MIC (μg/mL) | Antitubercular Activity MIC (μg/mL) | |||
|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P. aeruginosa | M. smegmatis | |
| 3a | 200 | 400 | 50 | 100 | - |
| 3b | 100 | 200 | 100 | 200 | - |
| 3c | 200 | 200 | 100 | 100 | - |
| 3d | 200 | 400 | 100 | 100 | - |
| 3e | 400 | 800 | 200 | 200 | - |
| 3f | 400 | 800 | 100 | 200 | - |
| 4a | 400 | 800 | 400 | 400 | 100 |
| 4b | 400 | 800 | 200 | 400 | 100 |
| 4c | 800 | - | 400 | - | 50 |
| 4d | - | - | 400 | - | 50 |
| 5a | 100 | 100 | 100 | 100 | - |
| 5b | 50 | 100 | 50 | 50 | - |
| 6a | 100 | 100 | 100 | 400 | 800 |
| 6b | 200 | 200 | 200 | 400 | - |
| CIP | 25 | 100 | 25 | 50 | / |
| RIP | / | / | / | / | 25 |
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of 1a–c
3.2.2. General Procedure for the Synthesis of Compounds 2a–f
3.2.3. General Procedure for the Synthesis of Compounds 3a–f
3.2.4. General Procedure for the Synthesis of Compounds 4a–d
3.2.5. General Procedure for the Synthesis of Compounds 5a–b
3.2.6. General Procedure for the Synthesis of Compounds 6a–b
3.3. Bioassays
4. Conclusions
Supplementary Material
Acknowledgments
Author Contributions
Conflicts of Interest
References
- El-Bayouki, K.A.; Basyouni, W.M. Thiazolopyrimidines without bridge-head nitrogen: Thiazolo [4,5-d] pyrimidines. J. Sulfur Chem. 2010, 31, 551–590. [Google Scholar] [CrossRef]
- Nagarajaiah, H.; Khazi, I.; Begum, N.S. Synthesis, characterization and biological evaluation of thiazolopyrimidine derivatives. J. Chem. Sci. 2012, 124, 847–855. [Google Scholar] [CrossRef]
- Tozkoparan, B.; Ertan, M.; Krebs, B.; Läge, M.; Kelicen, P.; Demirdamar, R. Condensed heterocyclic compounds: Synthesis and antiinflammatory activity of novel thiazolo [3,2-a] pyrimidines. Arch. Pharm. 1998, 331, 201–206. [Google Scholar] [CrossRef]
- Tozkoparan, B.; Ertan, M.; Kelicen, P.; Demirdamar, R. Synthesis and anti-inflammatory activities of some thiazolo [3,2-a] pyrimidine derivatives. Farmaco 1999, 54, 588–593. [Google Scholar] [CrossRef]
- Jeanneau-Nicolle, E.; Benoit-Guyod, M.; Namil, A.; Leclerc, G. New thiazolo [3,2-a] pyrimidine derivatives, synthesis and structure-activity relationships. Eur. J. Med. Chem. 1992, 27, 115–120. [Google Scholar] [CrossRef]
- Pan, B.; Huang, R.; Zheng, L.; Chen, C.; Han, S.; Qu, D.; Zhu, M.; Wei, P. Thiazolidione derivatives as novel antibiofilm agents: Design, synthesis, biological evaluation, and structure-activity relationships. Eur. J. Med. Chem. 2011, 46, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.; Abdel-Gawad, S.; El-Gaby, M. Synthesis and evaluation of some new fluorinated hydroquinazoline derivatives as antifungal agents. Farmaco 2000, 55, 249–255. [Google Scholar] [CrossRef]
- Mohamed, S.F.; Flefel, E.M.; Amr, A.E.G.E.; El-Shafy, D.N.A. Anti-HSV-1 activity and mechanism of action of some new synthesized substituted pyrimidine, thiopyrimidine and thiazolopyrimidine derivatives. Eur. J. Med. Chem. 2010, 45, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Maddila, S.; Damu, G.; Oseghe, E.; Abafe, O.; Rao, C.V.; Lavanya, P. Synthesis and biological studies of novel biphenyl-3,5-dihydro-2H-thiazolopyrimidines derivatives. J. Korean Chem. Soc. 2012, 56, 334–340. [Google Scholar] [CrossRef]
- Flefel, E.; Salama, M.; El-Shahat, M.; El-Hashash, M.; El-Farargy, A. A novel synthesis of some new pyrimidine and thiazolopyrimidine derivatives for anticancer evaluation. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 1739–1756. [Google Scholar] [CrossRef]
- Al-Omary, F.A.; Hassan, G.S.; El-Messery, S.M.; El-Subbagh, H.I. Substituted thiazoles V. Synthesis and antitumor activity of novel thiazolo [2,3-b] quinazoline and pyrido [4,3-d] thiazolo [3,2-a] pyrimidine analogues. Eur. J. Med. Chem. 2012, 47, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Danel, K.; Pedersen, E.B.; Nielsen, C. Synthesis and anti-HIV-1 activity of novel 2,3-dihydro-7 H-thiazolo [3,2-a] pyrimidin-7-ones. J. Med. Chem. 1998, 41, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Balkan, A.; Uma, S.; Ertan, M.; Wiegrebe, W. Thiazolo [3,2-α]-pyrimidine derivatives as calcium antagonists. Pharmazie 1992, 47, 687–688. [Google Scholar] [PubMed]
- Geist, J.G.; Lauw, S.; Illarionova, V.; Illarionov, B.; Fischer, M.; Gräwert, T.; Rohdich, F.; Eisenreich, W.; Kaiser, J.; Groll, M. Thiazolopyrimidine inhibitors of 2-methylerythritol 2,4-cyclodiphosphate synthase (IspF) from mycobacterium tuberculosis and plasmodium falciparum. ChemMedChem 2010, 5, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.; Adam, G.; Kolczewski, S.; Mutel, V.; Woltering, T. Structure-activity relationships of substituted 5H-thiazolo [3,2-a] pyrimidines as group 2 metabotropic glutamate receptor antagonists. Bioorg. Med. Chem. Lett. 1999, 9, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Awadallah, F.M. Synthesis, pharmacophore modeling, and biological evaluation of novel 5H-thiazolo [3,2-a] pyrimidin-5-one derivatives as 5-HT2A receptor antagonists. Sci. Pharm. 2008, 76, 415–438. [Google Scholar] [CrossRef]
- Kolb, S.; Mondésert, O.; Goddard, M.L.; Jullien, D.; Villoutreix, B.O.; Ducommun, B.; Garbay, C.; Braud, E. Development of novel thiazolopyrimidines as CDC25B phosphatase inhibitors. ChemMedChem 2009, 4, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shang, R.; Shi, L.; Wan, D.C. C.; Lin, H. Synthesis and biological evaluation of 7H-thiazolo [3,2-b]-1,2,4-triazin-7-one derivatives as dual binding site acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2014, 81, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Cai, J. Structural chemistry and properties of metal arenesulfonates. Coord. Chem. Rev. 2004, 248, 1061–1083. [Google Scholar] [CrossRef]
- Khan, R.H.; Rastogi, R.C. Condensed heterocycles: Synthesis and antifungal activity of. π-deficient pyrimidines linked with. π-rich heterocycles. J. Agric. Food Chem. 1991, 39, 2300–2303. [Google Scholar] [CrossRef]
- Veretennikov, E.; Pavlov, A. Synthesis of 5H-[1,3] thiazolo [3,2-a] pyrimidin-5-one derivatives. Russ. J. Org. Chem. 2013, 49, 575–579. [Google Scholar] [CrossRef]
- Janietz, D.; Goldmann, B.; Rudorf, W.D. Chlormethyl-substituierte Heterocyclen aus Chlortetrolsäuremethylester. J. Prakt. Chem. Chem. Ztg. 1988, 330, 607–616. [Google Scholar] [CrossRef]
- Doria, G.; Passarotti, C.; Sala, R.; Magrini, R.; Sberze, P.; Tibolla, M.; Ceserani, R.; Arcari, G.; Castello, R.; Toti, D. 7-trans-(2-pyridylethenyl)-5H-thiazolo [3,2-a] pyrimidine-5-ones: Synthesis and pharmacological activity. Farmaco 1985, 40, 885–894. [Google Scholar]
- Allen, C.; Beilfuss, H.; Burness, D.; Reynolds, G.; Tinker, J.; VanAllan, J. The structure of certain polyazaindenes. II. The product from ethyl acetoacetate and 3-amino-1,2,4-triazole. J. Org. Chem. 1959, 24, 787–793. [Google Scholar] [CrossRef]
- Dhapalapur, M.; Sabnis, S.; Deliwala, C. Potential anticancer agents. II. Schiff bases from benzaldehyde nitrogen mustards. J. Med. Chem. 1968, 11, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Raval, J.P.; Naik, B.N.; Desai, K.R. A convenient, rapid microwave-assisted synthesis of 2-substituted phenyl-2,3-dihydrobenzo [B][1,4] Thiazepine-3-carboxamide derivatives and its antimicrobial activity. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 255–267. [Google Scholar] [CrossRef]
- Fujiwara, M.; Inoi, T. Syntheses and anti-inflammatory activity of novel oximes and O-acyloximes. Chem. Pharm. Bull 1992, 40, 2419–2422. [Google Scholar]
- Bondock, S.; El-Azab, H.; Kandeel, E.E.M.; Metwally, M.A. Efficient synthesis of new functionalized 2-(hetaryl) thiazoles. Synth. Commun. 2013, 43, 59–71. [Google Scholar] [CrossRef]
- Parekh, N.M.; Maheria, K.C. Antituberculosis and antibacterial evaluations of some novel phenyl pyrazolone-substituted 1H-benzo [g] pyrazolo [3,4-b] quinoline-3-ylamine derivatives. Med. Chem. Res. 2012, 21, 4168–4176. [Google Scholar] [CrossRef]
- Youssef, M.M.; Youssef, A.M. Reactions with 2-thiothymine; selective cyclization of S-substituted 2-thiothymine. Phosphorus Sulfur Silicon Relat. Elem. 2003, 178, 67–81. [Google Scholar] [CrossRef]
- El-Emary, T.; Abdel-Mohsen, S.A. Synthesis and antimicrobial activity of some new 1, 3-diphenylpyrazoles bearing pyrimidine, pyrimidinethione, thiazolopyrimidine, triazolopyrimidine, thio- and alkylthiotriazolop-yrimidinone moieties at the 4-position. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2459–2474. [Google Scholar] [CrossRef]
- Karthikeyan, M.S. Synthesis and antimicrobial studies of thiazolotriazinones. Eur. J. Med. Chem. 2010, 45, 5039–5043. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, M.S.; Holla, B.S.; Kumari, N.S. Synthesis and antimicrobial studies of novel dichlorofluorophenyl containing aminotriazolothiadiazines. Eur. J. Med. Chem. 2008, 43, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kumar, P.; de Clercq, E.; Balzarini, J.; Pannecouque, C.; Dewan, S.K.; Narasimhan, B. 4-[1-(Substituted aryl/alkyl carbonyl)-benzoimidazol-2-yl]-benzenesulfonic acids: Synthesis, antimicrobial activity, QSAR studies, and antiviral evaluation. Eur. J. Med. Chem. 2010, 45, 5985–5997. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.I.; Ashida, N.; Nagamatsu, T. Antitumor studies. Part 4: Design, synthesis, antitumor activity, and molecular docking study of novel 2-substituted 2-deoxoflavin-5-oxides, 2-deoxoalloxazine-5-oxides, and their 5-deaza analogs. Bioorg. Med. Chem. 2008, 16, 922–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogimori, T.; Emerson, C.; Braverman, L.; Wu, C.; Gambino, J.; Wright, G. Synthesis of 6-anilino-2-thiouracils and their inhibition of human placenta iodothyronine deiodinase. J. Med. Chem. 1985, 28, 1692–1694. [Google Scholar] [CrossRef] [PubMed]
- Hübsch, W.; Pfleiderer, W. Pteridines. Part LXXXVII. Synthesis and properties of 8-substituted 2-thiolumazines. Helv. Chim. Acta 1988, 71, 1379–1391. [Google Scholar] [CrossRef]
- Wang, Y.; Damu, G.L.; Lv, J.S.; Geng, R.X.; Yang, D.C.; Zhou, C.H. Design, synthesis and evaluation of clinafloxacin triazole hybrids as a new type of antibacterial and antifungal agents. Bioorg. Med. Chem. Lett. 2012, 22, 5363–5366. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Hoerger, E.; Mason, S. Simple pyrimidines. Part II. 1: 2-dihydro-1-methylpyrimidines and the configuration of the N-methyluracils. J. Chem. Soc. 1955. [Google Scholar]
- Andrew, H.F.; Bradsher, C.K. A new synthesis of thiazolo[3,2-a] pyrimidinones. J. Heterocycl. Chem. 1967, 4, 577–581. [Google Scholar] [CrossRef]
- Abdel-Mohsen, H.T.; Conrad, J.; Beifuss, U. Laccase-catalyzed domino reaction between catechols and 6-substituted 1,2,3,4-tetrahydro-4-oxo-2-thioxo-5-pyrimidinecarbonitriles for the synthesis of pyrimidobenzothiazole derivatives. J. Org. Chem. 2013, 78, 7986–8003. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, J.; Pullman, B. Taulomerism and electronic structure of biological pyrimidines. Adv. Heterocycl. Chem. 1975, 18, 199–335. [Google Scholar]
- Mizutani, M.; Sanemitsu, Y.; Tamaru, Y.; Yoshida, Z. Palladium-catalyzed polyhetero-Claisen rearrangement of 2-(allylthio) pyrimidin-4(3H)-ones. J. Org. Chem. 1985, 50, 764–768. [Google Scholar] [CrossRef]
- Cox, B.G.; de Maria, P.; Guerzoni, L. Aminoketone enolisation: Influence of increasing chain length on intramolecular catalysis. J. Chem. Soc. Perkin Trans. 2 1988. [Google Scholar] [CrossRef]
- Chiang, Y.; Kresge, A.; Meng, Q.; More O’Ferrall, R.; Zhu, Y. Keto-enol/enolate equilibria in the isochroman-4-one system. Effect of a β-oxygen substituent. J. Am. Chem. Soc. 2001, 123, 11562–11569. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.; Desai, K.R. Microbial screening of novel synthesized formazans having amide linkages. J. Heterocycl. Chem. 2006, 43, 1083–1089. [Google Scholar] [CrossRef]
- Bakavoli, M.; Raissi, H.; Tajabadi, J.; Karimi, M.; Davoodnia, A.; Nikpour, M. Investigation into the regioisomeric composition of some fused pyrimidines: 1H-NMR and theoretical studies. J. Sulfur Chem. 2008, 29, 25–30. [Google Scholar] [CrossRef]
- Schäfer, H.; Jablokoff, H.; Hentschel, M.; Gewald, K. 2-Arylamino-thiophen-3-carbonsäurederivate. J. Prakt. Chem. Chem. Ztg. 1984, 326, 917–923. [Google Scholar] [CrossRef]
- Schäfer, H.; Gewald, K.; Bellmann, P.; Gruner, M. Synthese und reaktionen von 2-arylhydrazono-2-cyan-N,N-dialkyl-acetamidinen. Monatsh. Chem. 1991, 122, 195–207. [Google Scholar] [CrossRef]
- Angioni, S.; Villa, D.C.; Dal Barco, S.; Quartarone, E.; Righetti, P.P.; Tomasi, C.; Mustarelli, P. Polysulfonation of PBI-based membranes for HT-PEMFCs: A possible way to maintain high proton transport at a low H3PO4 doping level. J. Mater. Chem. A 2014, 2, 663–671. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Yang, Y.K.; Gabrielsen, B.; Marquet, J. Pyrylium-mediated transformations of natural products. Part 3. Synthesis of water-soluble pyrylium salts and their preparative reactions with amines. J. Chem. Soc. Perkin Trans. 2 1984, 857–866. [Google Scholar] [CrossRef]
- Coates, J.; Sammes, P.G.; West, R.M. Enhancement of luminescence of europium (III) ions in water by use of synergistic chelation. Part 1.1: 1 and 2:1 complexes. J. Chem. Soc. Perkin Trans. 2 1996, 1275–1282. [Google Scholar] [CrossRef]
- Hübsch, W.; Pfleiderer, W. Pteridines. Part XLI. Synthesis and properties of 6,7,8-trimethyl-4-thiolumazine. Helv. Chim. Acta 1989, 72, 738–743. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1a–c, 2a–f, 5a–b, 6a–b are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, D.; Zhang, Z.-H.; Chen, Y.; Yan, X.-J.; Zou, L.-J.; Wang, Y.-X.; Liu, X.-Q. Synthesis, Antibacterial and Antitubercular Activities of Some 5H-Thiazolo[3,2-a]pyrimidin-5-ones and Sulfonic Acid Derivatives. Molecules 2015, 20, 16419-16434. https://doi.org/10.3390/molecules200916419
Cai D, Zhang Z-H, Chen Y, Yan X-J, Zou L-J, Wang Y-X, Liu X-Q. Synthesis, Antibacterial and Antitubercular Activities of Some 5H-Thiazolo[3,2-a]pyrimidin-5-ones and Sulfonic Acid Derivatives. Molecules. 2015; 20(9):16419-16434. https://doi.org/10.3390/molecules200916419
Chicago/Turabian StyleCai, Dong, Zhi-Hua Zhang, Yu Chen, Xin-Jia Yan, Liang-Jing Zou, Ya-Xin Wang, and Xue-Qi Liu. 2015. "Synthesis, Antibacterial and Antitubercular Activities of Some 5H-Thiazolo[3,2-a]pyrimidin-5-ones and Sulfonic Acid Derivatives" Molecules 20, no. 9: 16419-16434. https://doi.org/10.3390/molecules200916419
APA StyleCai, D., Zhang, Z. -H., Chen, Y., Yan, X. -J., Zou, L. -J., Wang, Y. -X., & Liu, X. -Q. (2015). Synthesis, Antibacterial and Antitubercular Activities of Some 5H-Thiazolo[3,2-a]pyrimidin-5-ones and Sulfonic Acid Derivatives. Molecules, 20(9), 16419-16434. https://doi.org/10.3390/molecules200916419