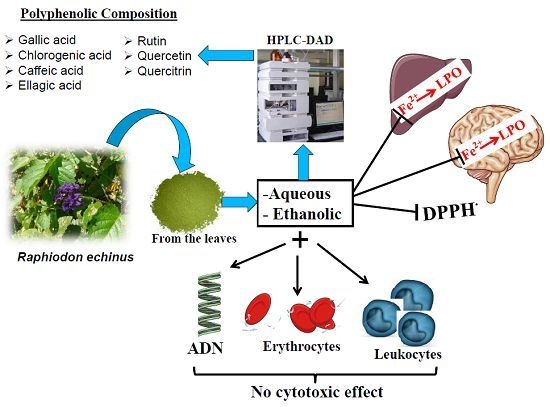
Polyphenolic Composition and Evaluation of Antioxidant Activity, Osmotic Fragility and Cytotoxic Effects of Raphiodon echinus (Nees & Mart.) Schauer
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC Characterization of the Polyphenolic Constituents of Aqueous and Ethanolic Extracts of R. echinus Leaves

| Time | Sequence of Addition | Reading at 510 nm |
|---|---|---|
| 0 min | Extract (30–120 μg/mL) | - |
| FeSO4 (110 μM) | ||
| 10 min | Ortho-phenanthroline (0.25%) | - |
| 10 min | Immediately after mixing with | First reading (0 min) |
| Ortho-phenanthroline | ||
| 20 min | - | Second reading (10 min) |
| 30 min | - | Third reading (20 min) |
| 30 min | Ascorbic acid (AA, final concentration, 5 mM) | - |
| 35 min | - | First reading after AA (25 min) |
| 40 min | - | Second reading after AA (30 min) |
| 50 min | - | Third reading after AA (40 min) |
| Compounds | Aqueous Extract/mg·g−1 (%) | Ethanolic Extract/mg·g−1 (%) | LOD/μg·mL−1 | LOQ/μg·mL−1 |
|---|---|---|---|---|
| Gallic acid | 11.59 ± 0.01 a (1.15) | 25.03 ± 0.01 a (2.50) | 0.011 | 0.037 |
| Chlorogenic acid | 25.07 ± 0.02 b (2.50) | 31.94 ± 0.02 b (3.19) | 0.009 | 0.035 |
| Caffeic acid | 40.19 ± 0.02 c (4.01) | 76.45 ± 0.02 c (7.64) | 0.026 | 0.090 |
| Ellagic acid | 63.18 ± 0.01 d (6.31) | 79.13 ± 0.03 c (7.91) | 0.017 | 0.056 |
| Rutin | 26.50 ± 0.03 b (2.65) | 35.84 ± 0.02 b (3.58) | 0.024 | 0.080 |
| Quercitrin | - | 7.12 ± 0.01 d (0.81) | 0.035 | 0.118 |
| Quercetin | 9.87 ± 0.03 a (0.98) | 12.37 ± 0.01 e (1.23) | 0.019 | 0.063 |
2.2. Antioxidant Activity
2.2.1. Scavenging Effect of R. echinus Extracts on DPPH Radical

| R. echinus | ||
|---|---|---|
| Aqueous Extract | Ethanolic Extract | |
| Total phenolics (mg GAE/g dry extract) | 173.0 ± 0.07 | 389.1 ± 0.04 |
2.2.2. Effect of R. echinus Extracts on Fe2+ Induced Lipid Peroxidation (LPO) in the Rat Brain and Liver Homogenates


2.2.3. Iron Chelating Potential of R. echinus Extracts

2.3. Cytotoxicity Effect of R. echinus Extracts on Human Leukocytes


2.4. Effect of R. echinus Extracts on Osmotic Fragility of Human Erythrocytes

2.5. Effect of R. echinus Extracts on DNA Damage

3. Experimental Section
3.1. Chemicals
3.2. Plant Material
3.3. Quantification of Compounds by HPLC-DAD
3.4. Determination of Total Phenolics
3.5. Antioxidant Activity
3.5.1. DPPH Radical Scavenging Activity
3.5.2. Production of TBARS from Animal Tissues
3.5.3. Iron Chelating Activity of R. echinus Extracts
3.6. Blood Collection and Preparation of Human Leukocytes and Erythrocytes
3.7. Determination of Erythrocytes Osmotic Fragility
3.8. Genotoxicity by the Comet Assay
3.9. Cytotoxicity by the Trypan Blue Exclusion Method (Cell Viability)
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmeiser, H.H.; Stiborova, M.; Arlt, V.M. Chemical and molecular basis of the carcinogenicity of Aristolochia plants. Curr. Opin. Drug Discov. Dev. 2009, 12, 141–148. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Anantha, N.D. Approaches to pre-formulation R and d for phytopharmaceuticals emanating from herb based traditional Ayurvedic processes. J. Ayurveda Integr. Med. 2013, 4, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Waczuk, E.P.; Kamdem, J.P.; Abolaji, A.O.; Meinerz, D.F.; Caeran Bueno, D.; do Nascimento Gonzaga, T.K.S.; do Canto Dorow, T.S.; Boligon, A.A.; Athayde, M.L.; da Rocha, J.B.T.; et al. Euphorbia tirucalli aqueous extract induces cytotoxicity, genotoxicity and changes in antioxidant gene expression in human leukocytes. Toxicol. Res. 2015, 4, 739–748. [Google Scholar]
- Vásquez, G.D.; Harley, R.M. Flora de Grão-Mogol, Minas Gerais: Labiatae. Bol. Bot. Univ. São Paulo 2004, 22, 193–204. [Google Scholar] [CrossRef]
- Souza, A.A.; Rodrigues, S.A. Antimicrobial activity of essential oil of Raphiodon echinus (Nees. e Mart) Shauer. Rev. Biol. Farm. 2012, 7, 12–17. [Google Scholar]
- Menezes, F.S.; Kaplan, M.A.K.; Cardoso, G.L.C.; Pereira, N.A. Phytochemical and pharmacological studies on Rhaphiodon echinus. Fitoterapia 1998, 69, 459–460. [Google Scholar]
- Kehrer, J.P.; Klotz, L. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef] [PubMed]
- Ambali, S.F.; Abubakar, M.; Shittu, M.; Yaqub, L.S.; Anafi, S.B.; Abdullahi, A. Chlorpyrifos induced alteration of haematological parameters in Wistar rats: Ameriolative effect of zinc. Res. J. Environ. Toxicol. 2010, 4, 55–66. [Google Scholar]
- Fetoui, H.; Gdoura, R. Synthetic pyrethroid increases lipid and protein oxidation and induces glutathione depletion in the cerebellum of adult rats: Ameliorative effect of vitamin C. Hum. Exp. Toxicol. 2012, 31, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.M.; Cheung, P.C.K.; Ooi, V.E.C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Kotássková, E.; Sumczynski, D.; Mlcek, J.; Valásek, P. Determination of free and bound phenolics using HPLC-DAD antioxidante activity and in vitro digestibility of Eragrostis tef. J. Food Compos. Anal. 2016, 46, 15–21. [Google Scholar] [CrossRef]
- Eugene, D.W. The hazards of iron loading. Metallomics 2010, 2, 732–740. [Google Scholar]
- Zecca, L.; Casella, L.; Albertini, A.; Bellei, C.; Zucca, F.A.; Engelen, M.; Zadlo, A.; Szewczyk, G.; Zareba, M.; Sarna, T. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J. Neurochem. 2008, 106, 1866–1875. [Google Scholar] [PubMed]
- Becerril-Ortega, J.; Bordji, K.; Fréret, T.; Rush, T.; Buisson, A. Iron overload accelerates neuronal amyloid-β production and cognitive impairment in transgenic mice model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Oliveira, C.S.; Noreen, H.; Kamdem, J.P.; Nogueira, C.W.; Rocha, J.B.T. Organoselenium compounds as potential neuroprotective therapeutic agents. Curr. Org. Chem. 2016, 20, 218–231. [Google Scholar] [CrossRef]
- Chikezie, A.R.; Akuwudike, C.M. Membrane stability and methaemoglobin content of human erythrocytes incubated in aqueous leaf extract of Nicotiana tabacum. Free Radic. Antioxid. 2012, 2, 56–61. [Google Scholar] [CrossRef]
- Pistón, M.; Machado, I.; Branco, C.S.; Cesio, V.; Heinzen, H.; Ribeiro, D.; Fernandes, E.; Chisté, R.C.; Freita, M. Infusion, decoction and of leaves from artichoke are effective scavengers of physiologically relevant ROS and RNS. Food Res. Int. 2014, 64, 150–156. [Google Scholar] [CrossRef]
- Iwahashi, H. Inhibitory effects of caffeic acid on free-radical formation. In Coffee in Health and Disease prevention; Preedy, V.R., Ed.; Elsevier Inc.: Atlanta, GA, USA, 2014; Volume 88, pp. 803–812. [Google Scholar]
- Oboh, G.; Puntel, R.L.; Rocha, J.B.T. Hot pepper (Capsicum annuum, tepin & Capsicum Chinese, habanero) prevents Fe2+-induced lipid peroxidation in brain—In vitro. Food Chem. 2007, 102, 178–185. [Google Scholar]
- Stoddart, M.J. Cell viability assays: Introduction. Methods Mol. Biol. 2011, 740, 1–6. [Google Scholar] [PubMed]
- He, J.; Lin, J.; Li, J.; Zhang, J.H.; Sun, X.M.; Zeng, C.M. Dual effects of Ginkgo biloba leaf extract on human red blood cells. Basic Clin. Pharmacol. Toxicol. 2009, 104, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, L.; Maktouf, S.; Kallel, C.; Ellouze Chaabouni, S.; Boudawara, T.; Zeghal, N. Disruption of erythrocyte antioxidant defense system, hematological parameters, induction of pro-inflammatory cytokines and DNA damage in liver of co-exposed rats to aluminiun and acrylamide. Chem. Biol. Interact. 2015, 236, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Tataranno, M.L.; Stazzoni, G.; del Vecchio, A.; Buonocore, G. Oxidative injury in neonatal erythrocytes. J. Matern. Fetal. Neonatal. Med. 2012, 25, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.; Lang, F. Mechanisms and pathophysiological significance of eryptosis, the suicidal erythrocyte death. Semin. Cell. Dev. Biol. 2015, 39, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, J.D. In vitro hemolysis of human erythrocytes by plant extracts with antiplasmodial activity. J. Ethnopharmacol. 2001, 74, 239–243. [Google Scholar] [CrossRef]
- Kamdem, J.P.; Adeniran, A.; Boligon, A.A.; Klimaczewski, C.V.; Elekofehinti, O.O.; Hassan, W.; Ibrahim, M.; Waczuk, E.P.; Meinerz, D.F.; Athayde, M.L. Antioxidant activity, genotoxicity and cytotoxicity evaluation of lemon balm (Melissa officinalis L.) ethanolic extract: Its potential role in neuroprotection. Ind. Crop. Prod. 2013, 51, 26–34. [Google Scholar] [CrossRef]
- Roy, P.; Mukherjee, A.; Giri, S. Evaluation of genetic damage in tobacco and arsenic exposed population of Southern Assam, India using buccal cytome and comet assay. Ecotoxicol. Environ. Saf. 2015, 124, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.M.; Miller, J.E.; Armstrong, M.J.; Bean, C.L.; Skopek, T.R.; Nichols, W.W. DNA synthesis inhibition as an indirect mechanism of chromosome aberrations: Comparison of DNA-reactive and non-DNA-reactive clastogens. Mutat. Res. 1998, 400, 169–186. [Google Scholar] [CrossRef]
- Galloway, S.M. Cytotoxicity and chromosome aberrations in vitro: Experience in industry and the case for an upper limit on toxicity in the aberration assay. Environ. Mol. Mutagen. 2000, 35, 191–201. [Google Scholar] [CrossRef]
- Pereira, R.P.; Boligon, A.A.; Appel, A.S.; Fachinetto, R.; Ceron, C.S.; Tanus-Santos, J.E.; Athayde, M.L.; Rocha, J.B.T. Chemical composition, antioxidant and anticholinesterase activity of Melissa officinalis. Ind. Crops Prod. 2014, 53, 34–45. [Google Scholar] [CrossRef]
- Kamdem, J.P.; Stefanello, S.T.; Boligon, A.A.; Wagner, C.; Kade, I.J.; Pereira, R.P.; Preste, A.S.; Roos, D.H.; Waczuk, E.P.; Appel, A.S.; et al. In vitro antioxidant activity of stem bark of Trichilia catigua Adr. Juss. Acta Pharm. 2012, 62, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Boligon, A.A.; Pereira, R.P.; Feltrin, A.C.; Machado, M.M.; Janovik, V.; Rocha, J.B.T.; Athayde, M.L. Antioxidant activities of flavonol derivatives from the leaves and stem bark of Scutia buxifolia Reiss. Bioresour. Technol. 2009, 100, 6592–6598. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Barbosa-Filho, V.M.; Waczuk, E.P.; Kamdem, J.P.; Lacerda, S.R.; Martin da Costa, J.C.; Alencar de Menesez, I.R.; Abolaji, A.O.; Boligon, A.A.; Athayde, M.L.; Rocha, J.B.T.; et al. Phytochemical constituents, antioxidant activity, cytotoxicity and osmotic fragility effects of caju (Anacardium. microcarpum). Ind. Crop. Prod. 2014, 55, 280–288. [Google Scholar] [CrossRef]
- Collins, A.R. The comet assay for DNA damage and repair. Principles, application, and limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Mischell, B.B.; Shiingi, S.M. Selected Methods in Cellular Immunology; WH Freeman Company: New York, NY, USA, 1980; pp. 1–469. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, A.E.; Waczuk, E.P.; Roversi, K.; Da Silva, M.A.P.; Barros, L.M.; Da Cunha, F.A.B.; De Menezes, I.R.A.; Da Costa, J.G.M.; Boligon, A.A.; Ademiluyi, A.O.; et al. Polyphenolic Composition and Evaluation of Antioxidant Activity, Osmotic Fragility and Cytotoxic Effects of Raphiodon echinus (Nees & Mart.) Schauer. Molecules 2016, 21, 2. https://doi.org/10.3390/molecules21010002
Duarte AE, Waczuk EP, Roversi K, Da Silva MAP, Barros LM, Da Cunha FAB, De Menezes IRA, Da Costa JGM, Boligon AA, Ademiluyi AO, et al. Polyphenolic Composition and Evaluation of Antioxidant Activity, Osmotic Fragility and Cytotoxic Effects of Raphiodon echinus (Nees & Mart.) Schauer. Molecules. 2016; 21(1):2. https://doi.org/10.3390/molecules21010002
Chicago/Turabian StyleDuarte, Antonia Eliene, Emily Pansera Waczuk, Katiane Roversi, Maria Arlene Pessoa Da Silva, Luiz Marivando Barros, Francisco Assis Bezerra Da Cunha, Irwin Rose Alencar De Menezes, José Galberto Martins Da Costa, Aline Augusti Boligon, Adedayo Oluwaseun Ademiluyi, and et al. 2016. "Polyphenolic Composition and Evaluation of Antioxidant Activity, Osmotic Fragility and Cytotoxic Effects of Raphiodon echinus (Nees & Mart.) Schauer" Molecules 21, no. 1: 2. https://doi.org/10.3390/molecules21010002
APA StyleDuarte, A. E., Waczuk, E. P., Roversi, K., Da Silva, M. A. P., Barros, L. M., Da Cunha, F. A. B., De Menezes, I. R. A., Da Costa, J. G. M., Boligon, A. A., Ademiluyi, A. O., Kamdem, J. P., Rocha, J. B. T., & Burger, M. E. (2016). Polyphenolic Composition and Evaluation of Antioxidant Activity, Osmotic Fragility and Cytotoxic Effects of Raphiodon echinus (Nees & Mart.) Schauer. Molecules, 21(1), 2. https://doi.org/10.3390/molecules21010002