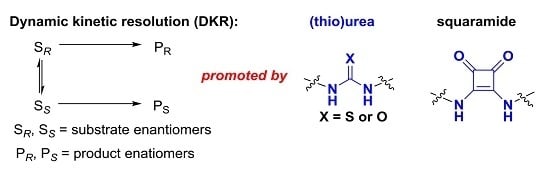
Recent Advances in Dynamic Kinetic Resolution by Chiral Bifunctional (Thio)urea- and Squaramide-Based Organocatalysts
Abstract
:1. Introduction
2. (Thio)urea Organocatalyst for DKR
2.1. Thio-Michael–Michael Cascade Reaction
2.2. Asymmetric Ring Opening of Azlactones
2.3. Atropo-Enantioselective Transesterification
3. Squaramide Organocatalyst for DKR
3.1. Enantioselective Selenofunctionalization Reactions
3.2. Asymmetric Ring Opening of Azlactones
3.3. Cascade Sulfur-Michael–Michael Reaction
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Farina, V.; Reeves, J.T.; Senanayake, C.H.; Song, J.J. Asymmetric synthesis of active pharmaceutical ingredients. Chem. Rev. 2006, 106, 2734–2793. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Solano, D.; Hoyos, P.; Hernáiz, M.J.; Alcántara, A.R.; Sánchez-Montero, J.M. Industrial biotransformation in the synthesis of building blocks leading to enantiopure drugs. Bioresour. Technol. 2012, 115, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Demain, A.L. Enzymes and bioconversion of industrial, pharmaceutical, and biotechnological significance. Org. Process Res. Dev. 2011, 15, 224–230. [Google Scholar] [CrossRef]
- Rajagopalan, A.; Kroutil, W. Biocatalytic reaction: Selected highlights. Mater. Today 2011, 14, 144–152. [Google Scholar] [CrossRef]
- Nestl, B.M.; Nebel, B.A.; Hauer, B. Recent progress in industrial biocatalysis. Curr. Opin. Chem. Biol. 2011, 15, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, A. Improved products and processes through biochemical engineering science. Biotechnol. J. 2011, 6, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, P.; Fu, W.; Jensen, J.S.; Woodley, J.M. Process consideration for the scale-up and implementation of biocatalysis. Food Bioprod. Process. 2010, 88, 3–11. [Google Scholar] [CrossRef]
- Lorenz, H.; Seidel-Morgenstern, A. Processes to Separate Enantiomers. Angew. Chem. Int. Ed. 2014, 53, 1218–1250. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Organocatalyzed dynamic kinetic resolution. Adv. Synth. Catal. 2011, 353, 659–676. [Google Scholar] [CrossRef]
- Keith, J.M.; Larrow, J.F.; Jacobsen, E.N. Practical Considerations in Kinetic Resolution Reactions. Adv. Synth. Catal. 2001, 343, 5–26. [Google Scholar] [CrossRef]
- Vedejs, E.; Jure, M. Efficiency in nonenzymatic kinetic resolution. Angew. Chem. Int. Ed. 2005, 44, 3974–4001. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.E.J.E.; Bull, S.D. Kinetic resolution strategies using non-enzymatic catalysts. Tetrahedron Asymmetry 2003, 14, 1407–1446. [Google Scholar] [CrossRef]
- El Gihani, M.T.; Williams, J.M.J. Dynamic kinetic resolution. Curr. Opin. Chem. Biol. 1999, 3, 11–15. [Google Scholar] [CrossRef]
- Kim, M.-J.; Ahn, Y.; Park, J. Dynamic kinetic resolutions and asymmetric transformations by enzymes coupled with metal catalysis. Curr. Opin. Biotechnol. 2002, 13, 578–587. [Google Scholar] [CrossRef]
- Pàmies, O.; Bäckvall, J.-E. Chemoenzymatic dynamic kinetic resolution. Trends Biotechnol. 2004, 22, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Martín-Matute, B.; Bäckvall, J.-E. Dynamic kinetic resolution catalyzed by enzymes and metals. Curr. Opin. Chem. Biol. 2007, 11, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Ko, S.-B.; Kim, M.-J.; Park, J. Racemization catalysts for the dynamic kinetic resolution of alcohols and amines. Coord. Chem. Rev. 2008, 252, 647–658. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, K.; Kim, M.-J.; Park, J. Chemoenzymatic dynamic kinetic resolution of alcohols and amines. Eur. J. Org. Chem. 2010, 999–1015. [Google Scholar] [CrossRef]
- Turner, N. Deracemisation methods. Curr. Opin. Chem. Biol. 2010, 14, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Azhar, M.A.; Krishnaji, T.; Malik, M.S.; Azeeza, S. Approaches based on enzyme mediated kinetic to dynamic kinetic resolution: A versatile route for chiral intermediates. Coord. Chem. Rev. 2008, 252, 569–592. [Google Scholar] [CrossRef]
- Warner, M.C.; Casey, C.P.; Bäckvall, J.E. Bifunctional Molecular Catalysis in Topics in Organometallic Chemistry; Springer: London, UK, 2011. [Google Scholar]
- Pellissier, H. Recent developments in dynamic kinetic resolution. Tetrahedron 2011, 67, 3769–3802. [Google Scholar] [CrossRef]
- Ahmed, M.; Kelly, T.; Ghanem, A. Applications of enzymatic and non-enzymatic methods to access enantiomerically pure compounds using kinetic resolution and racemisation. Tetrahedron 2012, 68, 6781–6802. [Google Scholar] [CrossRef]
- Hoyos, P.; Pace, V.; Alcántara, A.R. Dynamic kinetic resolution via hydrolase-metal combo catalysis in stereoselective synthesis of bioactive compounds. Adv. Synth. Catal. 2012, 354, 2585–2611. [Google Scholar] [CrossRef]
- Warner, M.C.; Bäckvall, J.-E. Mechanistic aspects on cyclopentadienylruthenium complexes in catalytic racemization of alcohols. Acc. Chem. Res. 2013, 46, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Denard, C.A.; Hartwig, J.F.; Zhao, H. Multistep one-pot reactions combining biocatalysts and chemical catalysts for asymmetric synthesis. ACS Catal. 2013, 3, 2856–2864. [Google Scholar] [CrossRef]
- Rachwalski, M.; Vermue, N.; Rutjes, F.P.J.T. Recent advances in enzymatic and chemical deracemisation of racemic compounds. Chem. Soc. Rev. 2013, 42, 9268–9282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramon, D.J.; Yus, M. In the arena of enantioselective synthesis, Titanium complexes wear the laurel wreath. Chem. Rev. 2006, 106, 2126–2208. [Google Scholar] [CrossRef] [PubMed]
- List, B. Introduction: Organocatalysis. Chem. Rev. 2007, 107, 5413–5415. [Google Scholar] [CrossRef]
- Moyano, A.; Rios, R. Asymmetric Organocatalytic Cyclization and Cycloaddition Reactions. Chem. Rev. 2011, 111, 4703–4832. [Google Scholar] [CrossRef] [PubMed]
- Dalko, P. Comprehensive Enantioselective Organocatalysis; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.P. Asymmetric and diastereoselective conjugate addition reaction: C–C bond formation at large scale. Org. Process Res. Dev. 2012, 16, 1258–1272. [Google Scholar] [CrossRef]
- Pihko, P.M. Hydrogen Bonding in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Doyle, A.G.; Jacobsen, E.N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 2007, 107, 5713–5743. [Google Scholar] [CrossRef] [PubMed]
- Palomo, C.; Oiarbide, M.; López, R. Asymmetric organocatalysis by chiral Bronsted bases: Implications and applications. Chem. Soc. Rev. 2009, 38, 632–653. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T. Stronger Brønsted acids. Chem. Rev. 2007, 107, 5744–5758. [Google Scholar] [CrossRef] [PubMed]
- Terada, M. Chiral phosphoric acids as versatile catalysts for enantioselective transformations. Synthesis 2010, 12, 1929–1982. [Google Scholar] [CrossRef]
- Maruoka, K. Asymmetric Phase Transfer Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Paull, D.H.; Abraham, C.J.; Scerba, M.T.; Alden-Danforth, E.; Lectka, T. Bifunctional asymmetric catalysis: Cooperative lewis acid/base systems. Acc. Chem. Res. 2008, 41, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Park, J.; Kim, M.J. Comprehensive Chirality; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Takemoto, Y. Recognition and activation by ureas and thioureas: Stereoselective reactions using ureas and thioureas as hydrogen-bonding donors. Org. Biomol. Chem. 2005, 3, 4299–4306. [Google Scholar] [CrossRef] [PubMed]
- Connon, S.J. Organocatalysis mediated by (Thio)urea derivatives. Chem. Eur. J. 2006, 12, 5418–5427. [Google Scholar] [CrossRef] [PubMed]
- Storer, R.I.; Aciro, C.; Jones, L.H. Squaramides: Physical properties, synthesis and applications. Chem. Soc. Rev. 2011, 40, 2330–2346. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, H.-B.; Dong, C. Applications of chiral squaramides: From asymmetric organocatalysis to biologically active compounds. Chem. Rec. 2016, 16, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Hu, X.-Q.; Li, Y.-H.; Xu, P.-F. Organocatalytic asymmetric multicomponent cascade reaction via 1,3-proton shift and [3+2] cycloaddition: An efficient strategy for synthesis of oxindole derivatives. Chem. Commun. 2013, 49, 7213–7215. [Google Scholar] [CrossRef] [PubMed]
- Badiola, E.; Fiser, B.; Gómez-Bengoa, E.; Mielgo, A.; Olaizola, I.; Urruzuno, I.; García, J.M.; Odriozola, J.M.; Razkin, J.; Oiarbide, M.; Palomo, C. Enantioselective construction of tetrasubstituted stereogenic carbons through Bronsted Base catalyzed Michael reaction: α′-hydroxy enones as key enoate equivalent. J. Am. Chem. Soc. 2014, 136, 17869–17881. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-M.; Shen, F.-F.; Zhang, F.-T.; Zhang, J.-L.; Wang, R. Catalytic asymmetric 1,2-addition of α-isothiocyanato phosphonates: Snthesis of chiral β-hydroxy- or β-amino-substituted α-amino phosphonic acid derivatives. Angew. Chem. Int. Ed. 2014, 53, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-L.; Du, D.-M. Chiral squaramide-catalyzed Michael/alkylation cascade reaction for the asymmetric synthesis of nitro-spirocyclopropanes. Eur. J. Org. Chem. 2015, 5350–5359. [Google Scholar] [CrossRef]
- Sigman, M.S.; Jacobsen, E.N. Schiff Base Catalysts for the Asymmetric Strecker Reaction Identified and Optimized from Parallel Synthetic Libraries. J. Am. Chem. Soc. 1998, 120, 4901–4902. [Google Scholar] [CrossRef]
- Malerich, J.P.; Hagihara, K.; Rawal, V.H. Chiral Squaramide Derivatives are Excellent Hydrogen Bond Donor Catalysts. J. Am. Chem. Soc. 2008, 130, 14416–14417. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jacobsen, E.N. Highly enantioselective direct conjugate addition of ketones to nitroalkenes promoted by a chiral primary amine-thiourea catalyst. J. Am. Chem. Soc. 2006, 128, 7170–7171. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. Bifunctional amine-squaramides: Powerful hydrogen-bonding organocatalysts for asymmetric domino/cascade reactions. Adv. Synth. Catal. 2015, 357, 253–281. [Google Scholar] [CrossRef]
- Held, F.E.; Tsogoeva, S.B. Asymmetric cycloaddition reactions catalyzed by bifunctional thiourea and squaramide organocatalysts: Recent advances. Catal. Sci. Technol. 2016, 6, 645–667. [Google Scholar] [CrossRef]
- Tsakos, M.; Kokotos, C.G. Primary and secondary amine-(thio)ureas and squaramides and their application in asymmetric organocatalysis. Tetrahedron 2013, 69, 10199–10222. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Wang, J.; Zu, L. Enantioselective organocatalytic tandem Michael-aldol reactions: One-pot synthesis of chiral thiochromenes. J. Am. Chem. Soc. 2006, 128, 10354–10355. [Google Scholar] [CrossRef] [PubMed]
- Rios, R.; Sundén, H.; Ibrahem, I.; Zhao, G.L.; Eriksson, L.; Córdova, A. Highly enantioselective synthesis of 2H-1-benzothiopyrans by a catalytic domino reaction. Tetrahedron Lett. 2006, 47, 8547–8551. [Google Scholar] [CrossRef]
- Zu, L.S.; Wang, J.; Li, H.; Xie, H.X.; Jiang, W.; Wang, W. Cascade Michael-aldol reactions promoted by hydrogen bonding mediated catalysis. J. Am. Chem. Soc. 2007, 129, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; E-Nunu, T.; Zu, L.; Jiang, W.; Wei, S.; Wang, W. One-pot approach to chiral chromenes via enantioselective organocatalytic domino oxa-Michael-Aldol reaction. Chem. Commun. 2007, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Hua, Q.-L.; Cheng, Y.; An, X.-L.; Yang, Q.-Q.; Chen, J.-R.; Xiao, W.-J. Organocatalytic asymmetric Sulfa-Michael/Michael addition reactions: A strategy for synthesis of highly substituted chromans with a quaternary stereocenter. Angew. Chem. Int. Ed. 2010, 49, 8379–8561. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Zheng, B.; Chen, J.; Peng, Y. Asymmetric synthesis of polysubstituted 4-amino- and 3,4-diaminochromanes with a chiral multifunctional organocatalyst. Org. Lett. 2012, 14, 2378–2381. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; An, J.; Zhang, X.-X.; Tan, F.; Chen, J.-R.; Xiao, W.-J. Catalytic asymmetric aza-Michael-Michael addition cascade: Enantioselective synthesis of polysubstituted 4-aminobenzopyrans. Org. Lett. 2011, 13, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Maerten, E.; Jørgensen, K.A. Asymmetric synthesis of highly functionalized tetrahydrothiophenes by organocatalytic domino reactions. J. Am. Chem. Soc. 2006, 128, 14986–14991. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zu, L.; Xie, H.; Wang, J.; Jiang, W.; Wang, W. Enantioselective organocatalytic double Michael addition reactions. Org. Lett. 2007, 9, 1833–1835. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.B.; Su, Y.; Zhu, H.-L.; Wang, G.-Y.; Xu, P.-F. Hydrogen-bond-mediated cascade reaction involving chalcones: Facile synthesis of enantioenriched trisubstituted tetrahydrothiophenes. Org. Lett. 2012, 14, 1090–1093. [Google Scholar] [CrossRef] [PubMed]
- Zu, L.; Xie, H.; Li, H.; Wang, J.; Jiang, W.; Wang, W. Chiral Amine Thiourea-Promoted Enantioselective Domino Michael-Aldol Reactions between 2-Mercaptobenzaldehydes and Maleimides. Adv. Synth. Catal. 2007, 349, 1882–1886. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Li, H.; Zu, L.; Wang, W. A highly stereoselective hydrogen-bond-mediated Michael-Michael cascade process through dynamic kinetic resolution. Angew. Chem. Int. Ed. 2008, 47, 4177–4179. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, Y.; Song, A.; Ji, Y.; Wang, W. Interplay of direct stereocontrol and dynamic kinetic resolution in a bifunctional amine thiourea catalyzed highly enantioselective cascade Michael-Michael reaction. Chem. Eur. J. 2011, 17, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Meninno, S.; Croce, G.; Lattanzi, A. Asymmetric synthesis of trisubstituted tetrahydrothiophenes bearing a quaternary stereocenter via double Michael reaction involving dynamic kinetic resolution. Org. Lett. 2013, 15, 3436–3439. [Google Scholar] [CrossRef] [PubMed]
- Breuer, M.; Ditrich, K.; Habicher, T.; Hauer, B.; Kebeler, M.; Stermer, R.; Zelinski, T. Industrial methods for the production of optically active intermediates. Angew. Chem. Int. Ed. 2004, 43, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.N. Stereoselective Biocatalysis; Marcel Dekker: New York, NY, USA, 2000; pp. 877–902. [Google Scholar]
- Jarvo, E.R.; Miller, S.J. Amino acids and peptides as asymmetric organocatalysts. Tetrahedron 2002, 58, 2481–2495. [Google Scholar] [CrossRef]
- Blaser, H.U.; Federsel, H.J. Asymmetric Catalysis on Industrial Scale; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Carter, H.E. Azlactones. Org. React. 1946, 3, 198–239. [Google Scholar]
- Berkessel, A.; Cleemann, F.; Mukherjee, S.; Mueller, T.N.; Lex, J. Highly efficient dynamic kinetic resolution of azlactones by urea-based bifunctional organocatalysts. Angew. Chem. Int. Ed. 2005, 44, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Berkessel, A.; Mukherjee, S.; Cleemann, F.; Mueller, T.N.; Lex, J. Second-generation organocatalysts for the highly enantioselective dynamic kinetic resolution of azlactones. Chem. Commun. 2005, 14, 1898–1900. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.B.; Rho, H.S.; Oh, J.S.; Nam, E.H.; Park, S.E.; Bae, H.Y.; Song, C.E. DOSY NMR for monitoring self-aggregation of bifunctional organocatalysts: Increasing enantioselectivity with decreasing catalyst concentration. Org. Biomol. Chem. 2010, 8, 3918–3922. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Rho, H.S.; Lee, J.W.; Lee, J.E.; Youk, S.H.; Chin, J.; Song, C.E. A highly reactive and enantioselective bifunctional organocatalyst for the mechanolytic desymmetrization of cyclic anhydrides: Prevention of catalyst aggregation. Angew. Chem. Int. Ed. 2008, 41, 7990–7993. [Google Scholar] [CrossRef]
- Rho, H.S.; Oh, J.S.; Lee, J.W.; Lee, J.Y.; Chin, J.; Song, C.E. Bifunctional organocatalyst for methanolytic desymmetrization of cyclic anhydrides. Chem. Commun. 2008, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-S.; Lee, J.-W.; Ryu, T.H.; Lee, J.H.; Song, C.E. Self-assocation free bifunctional thiourea organocatalysts: Synthesis of chiral α-amino acids via dynamic kinetic resolution of racemic azlactiones. Org. Biomol. Chem. 2012, 10, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, M.C.; Morgan, B.J.; Linton, E.C. Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling. Chem. Soc. Rev. 2009, 38, 3193–3207. [Google Scholar] [CrossRef] [PubMed]
- Noyori, R.; Takaya, H. BINAP: An efficient chiral element for asymmetric catalysis. Acc. Chem. Res. 1990, 23, 345–350. [Google Scholar] [CrossRef]
- Chen, Y.; Yekta, S.; Yudin, A.K. Modified BINOL ligands in asymmetric catalysis. Chem. Rev. 2003, 103, 3155–3212. [Google Scholar] [CrossRef] [PubMed]
- Terada, M. Binaphthol-derived phosphoric acid as a versatile catalyst for enantioselective carbon-carbon bond forming reactions. Chem. Commun. 2008, 20, 4097–4112. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Bronsted acid and metal catalysis: History and classification by mode of activation; Bronsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 2014, 114, 9047–9153. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Niizuma, S.; Kamikawa, T.; Suzuki, N.; Uozumi, Y. Catalytic asymmetric synthesis of axially chiral biaryls by palladium-catalyzed enantioposition-selective cross-coupling. J. Am. Chem. Soc. 1995, 117, 9101–9102. [Google Scholar] [CrossRef]
- Kakiuchi, F.; Le Gendre, P.; Yamada, A.; Ohtaki, H.; Murai, S. Atropselective alkylation of biaryl compounds by means of transition metal-catalyzed C-H/olefin coupling. Tetrahedron Asymmetry 2000, 11, 2647–2651. [Google Scholar] [CrossRef]
- Bhat, V.; Wang, S.; Stoltz, B.M.; Virgil, S.C. Asymmetric synthesis of QUINAP via Dynamic kinetic resolution. J. Am. Chem. Soc. 2013, 135, 16829–16832. [Google Scholar] [CrossRef] [PubMed]
- Ros, A.; Estepa, B.; Ramírez-Lopez, P.; Álvarez, E.; Fernandez, R.; Lassaletta, J.M. Dynamic kinetic cross-coupling strategy for the asymmetric synthesis of axially chiral heterobiaryls. J. Am. Chem. Soc. 2013, 135, 15730–15733. [Google Scholar] [CrossRef] [PubMed]
- Hazra, C.K.; Dherbassy, Q.; Wencel-Delord, J.; Colobert, F. Synthesis of axially chiral biaryls through sulfoxide-directed asymmetric mild C-H activation and dynamic kinetic resolution. Angew. Chem. Int. Ed. 2014, 53, 14091–14095. [Google Scholar] [CrossRef]
- Zheng, J.; You, S.-L. Construction of axial chirality by Rhodium-catalyzed asymmetric dehydrogenative Heck coupling of biaryl compounds with alkenes. Angew. Chem. Int. Ed. 2014, 53, 13244–13463. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, J.L.; Lim, D.; Miller, S.J. Dynamic kinetic resolution of biaryl atropisomers via peptide-catalyzed asymmetric bromination. Science 2010, 328, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, J.L.; Lim, D.; Barrett, K.T.; Miller, S.J. Synthesis of atropisomerically defined, highly substituted biaryl scaffolds through catalytic enantioselective bromination and regioseletive cross-coupling. Angew. Chem. Int. Ed. 2011, 50, 5125–5129. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Ichikawa, Y.; Kobayashi, M.; Shibata, Y.; Yamanaka, M.; Akiyama, T. Enantioselevtive synthesis of multisubstituted biaryl skeleton by chiral phosphoric acid catalyzed desymmetrization/kinetic resolution sequence. J. Am. Chem. Soc. 2013, 135, 3964–3970. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, S.; Wu, X.; Maruoka, K. Kinetic resolution of axially chiral 2-amino-1,1′-biaryls by Phase-Transfer-Catalyzed N-Allylation. Angew. Chem. Int. Ed. 2013, 52, 14200–14203. [Google Scholar] [CrossRef] [PubMed]
- De, C.K.; Pesciaioli, F.; List, B. Catalytic asymmetric benzidine rearrangement. Angew. Chem., Int. Ed. 2013, 52, 9293–9295. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Deng, J.; Sibi, M.P. Fluxionally chiral DMAP catalysts: Kinetic resolution of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 2014, 44, 12012–12015. [Google Scholar] [CrossRef]
- Yu, C.; Huang, H.; Li, X.; Zhang, Y.; Wang, W. Dynamic kinetic resolution of biaryl lactones via a chiral bifunctional amine thiourea-catalyzed highly atropo-enantioselective transesterification. J. Am. Chem. Soc. 2016, 138, 6956–6959. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Petasis, N.A. Selenium in Natural Product Synthesis; CIS: Philadelphia, PA, US, 1984. [Google Scholar]
- Liotta, D. Organoselenium Chemistry; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Back, T.G. Organoselenium Chemistry A Practical Approach; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Wirth, T. Topics in Current Chemistry: Organoselenium Chemistry; Springer: Heidelberg, Germany, 2000. [Google Scholar]
- Petragnani, N.; Stefani, H.A.; Valduga, C.J. Recent advances in selenocyclofunctionalization reactions. Tetrahedron 2001, 57, 1411–1448. [Google Scholar] [CrossRef]
- Ranganathan, S.; Muraleedharan, K.M.; Vaish, N.K.; Jayaraman, N. Halo- and selenolactonisation: The two major strategies for cyclofunctionalisation. Tetrahedron 2004, 60, 5273–5308. [Google Scholar]
- Wirth, T. Organoselenium Chemistry: Synthesis and Reactions; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Zhang, H.; Lin, S.; Jacobsen, E.N. Enantioselective selenocyclization via dynamic kinetic resolution of seleniranium ions by hydrogen-bond donor catalysts. J. Am. Chem. Soc. 2014, 136, 16485–16488. [Google Scholar] [CrossRef] [PubMed]
- Woong Lee, J.; Hi Ryu, T.; Suk Oh, J.; Yong Bae, H.; Bin Jang, H.; Eui Song, C. Self-association-free dimeric cinchona alkaloid organocatalysts: Unprecedented catalytic activity, enantioselectivity and catalyst recyclability in dynamic kinetic resolution of racemic azlactones. Chem. Commun. 2009, 46, 7224–7226. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-S.; Kim, K.I.; Song, C.E. Enantioselective synthesis of α-deuterium labeled chiral α-amino acids via dynamic kinetic resolution of racemic azlactones. Org. Biomol. Chem. 2011, 9, 7983–7985. [Google Scholar] [CrossRef] [PubMed]
- Afantitis, A.; Melagraki, G.; Sarimveis, H.; Koutentis, P.A.; Markopoulos, J.; Igglessi-Markopoulou, O. A novel QSAR model for predicting induction of apoptosis by 4-aryl-4H-chromenes. Bioorg. Med. Chem. 2006, 14, 6686–6694. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.; Monaco, L.P.; Desideri, N. Design, synthesis and in vitro evaluation of novel chroman-4-one chroman, and 2H-chromene derivatives as human rhinovirus capsid-binding inhibitors. Bioorg. Med. Chem. 2011, 19, 7357–7364. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Yamazaki, K.; Ikeshiro, Y.; Yamagishi, T.; Fujioka, T.; Mihashi, K.; Mizuki, K.; Cosentino, L.M.; Fowke, K.; Morris-Natschke, S.L.; Lee, K.-H. Isolation of rhododaurichchromanic acid B and the anti-HIV principles rhododaurichromanic acid A and rhododaurichchromenic acid from Rhododendron dauricum. Tetrahedron 2001, 57, 1559–1563. [Google Scholar] [CrossRef]
- Zu, L.; Zhang, S.; Xie, H.; Wang, W. Catalytic asymmetric oxa-Michael-Michael cascade for facile construction of chiral chromans via an aminal intermediate. Org. Lett. 2009, 11, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, Y.; Du, D.-M. Squaramide-tertiary amine catalyzed asymmetric cascade Sulfa-Michael/Michael addition via dynamic kinetic resolution: Access to highly functionalized chromans with three contiguous stereocenters. Org. Lett. 2013, 15, 1190–1193. [Google Scholar] [CrossRef] [PubMed]


















© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Hu, X.; Dong, X.-Q.; Zhang, X. Recent Advances in Dynamic Kinetic Resolution by Chiral Bifunctional (Thio)urea- and Squaramide-Based Organocatalysts. Molecules 2016, 21, 1327. https://doi.org/10.3390/molecules21101327
Li P, Hu X, Dong X-Q, Zhang X. Recent Advances in Dynamic Kinetic Resolution by Chiral Bifunctional (Thio)urea- and Squaramide-Based Organocatalysts. Molecules. 2016; 21(10):1327. https://doi.org/10.3390/molecules21101327
Chicago/Turabian StyleLi, Pan, Xinquan Hu, Xiu-Qin Dong, and Xumu Zhang. 2016. "Recent Advances in Dynamic Kinetic Resolution by Chiral Bifunctional (Thio)urea- and Squaramide-Based Organocatalysts" Molecules 21, no. 10: 1327. https://doi.org/10.3390/molecules21101327
APA StyleLi, P., Hu, X., Dong, X. -Q., & Zhang, X. (2016). Recent Advances in Dynamic Kinetic Resolution by Chiral Bifunctional (Thio)urea- and Squaramide-Based Organocatalysts. Molecules, 21(10), 1327. https://doi.org/10.3390/molecules21101327