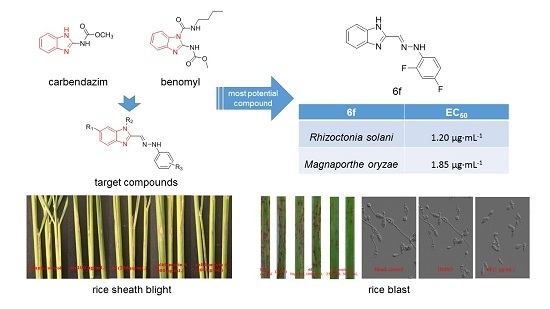
Synthesis and Biological Evaluation of Benzimidazole Phenylhydrazone Derivatives as Antifungal Agents against Phytopathogenic Fungi
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Compounds
2.2. Crystal Structure of Compound 6b
2.3. Antifungal Activities In Vitro
2.4. Inhibition of 6f on the Sclerotia Germination of R. solani
2.5. Protective Activity of 6f against Rice Sheath Blight (RSB) In Vivo
2.6. Inhibition of 6f on the Conidium Germination of M. oryzae
2.7. Protective Activity of 6f against Rice Blast (RB) In Vivo
3. Materials and Methods
3.1. Reagents and Analysis
3.2. Synthesis
3.2.1. Synthesis and Purification of Compound 2a–2d
3.2.2. Synthesis and Purification of Compound 3a–3d
3.2.3. Synthesis and Purification of Compound 5a–5s
3.2.4. Synthesis and Purification of Compound 6a–6ai
3.3. Crystallographic Study
3.4. Biological Assay
3.4.1. Antifungal Activity Assays in Vitro
3.4.2. Inhibition of 6f on the Sclerotia Germination of R. solani
3.4.3. Protective Activity of 6f against RSB In Vivo
3.4.4. Inhibition of 6f on the Conidium Germination of M. oryzae
3.4.5. Protective Activity of 6f against RB In Vivo
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Woolley, D.W. Some biological effects producted by benzimidazole and their reversal by purines. J. Biol. Chem. 1944, 152, 225–232. [Google Scholar]
- Madkour, H.; Farag, A.A.; Ramses, S.S.; Ibrahiem, N. Synthesis and fungicidal activity of new imidazoles from 2-(chloromethyl)-1H-benzimidazole. Phosphorus Sulfur 2006, 181, 255–265. [Google Scholar] [CrossRef]
- Singla, P.; Luxami, V.; Paul, K. Synthesis and in vitro evaluation of novel triazine analogues as anticancer agents and their interaction studies with bovine serum albumin. Eur. J. Med. Chem. 2016, 117, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Ma, S.T. Recent development of benzimidazole-containing antibacterial agents. ChemMedChem 2016, 11, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Kus, C.; Ayhan-Kilcigil, G.; Ozbey, S.; Kaynak, F.B.; Kaya, M.; Coban, T.; Can-Eke, B. Synthesis and antioxidant properties of novel N-methyl-1,3,4-thiadiazol-2-amine and 4-methyl-2H-1,2,4-triazole-3(4H)-thione derivatives of benzimidazole class. Bioorg. Med. Chem. 2008, 16, 4294–4303. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, T.; Gigantea, B.; Gilchrist, L. A short synthesis of phenanthro[2,3-d]imidazoles from dehydroabietic acid. Application of the methodology as a convenient route to benzimidazoles. Tetrahedron 2001, 57, 1793–1799. [Google Scholar] [CrossRef]
- Perez-Villanueva, J.; Santos, R.; Hernandez-Campos, A.; Giulianotti, M.A.; Castillo, R.; Medina-Franco, J.L. Structure-activity relationships of benzimidazole derivatives as antiparasitic agents: Dual activity-difference (DAD) maps. MedChemComm 2011, 2, 44–49. [Google Scholar] [CrossRef]
- Bi, C.W.; Qiu, J.B.; Zhou, M.G.; Chen, C.J.; Wang, J.X. Effects of carbendazim on conidial germination and mitosis in germlings of Fusarium graminearum and Botrytis cinerea. Int. J. Pset Manag. 2009, 55, 157–163. [Google Scholar]
- Tanejk, M.; Grover, R.K. Efficacy of benzimidazole and related fungicides against Rhizoctonia solain and R. bataticola. Ann. Appl. Biol. 1982, 100, 425–432. [Google Scholar] [CrossRef]
- Liu, L.X.; Tom, H. Estimating benzimidazole residues in thatch and turfgrass by bioassay. Pestic. Sci. 1996, 46, 139–143. [Google Scholar] [CrossRef]
- Zhu, L.F.; Hou, Z.; Zhou, K.; Tong, Z.B.; Kuang, Q.; Geng, H.L.; Zhou, L. Synthesis, bioactivity and structure-activity relationships of new 2-aryl-8-OR-3,4-dihydroisoquinolin-2-iums salts as potential antifungal agents. Bioorg. Med. Chem. Lett. 2016, 26, 2413–2417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dai, Z.C.; Qian, S.; Liu, J.; Xiao, Y.; Lu, A.; Zhu, H.L.; Wang, J.X.; Ye, Y.H. Design, synthesis, antifungal, and antioxidant activities of (E)-6-((2-phenylhydrazono)methyl)quinoxaline derivatives. J. Agric. Food Chem. 2014, 62, 9637–9643. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.C.; Chen, Y.F.; Zhang, M.; Li, S.K.; Yang, T.T.; Shen, L.; Wang, J.X.; Qian, S.; Zhu, H.L.; Ye, Y.H. Synthesis and antifungal activity of 1,2,3-triazole phenylhydrazone derivatives. Org. Biomol. Chem. 2015, 13, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.P.; Wathey, W.B. Benzimidazoles. Part 2. A new synthesis of [1,2,4] triazino [4,5-a] benzimidazol-1-ones. J. Chem. Res. 1984, 12, 384–385. [Google Scholar]
- Le, B.; Marie, T.; Wahl, H. Monoazo dye derivatives of 1,2-dimethylbenzimidazole. C. R. 1957, 245, 2058–2060. [Google Scholar]
- Eom, Y.W.; Oh, S.; Woo, H.B.; Ham, J.; Ahn, C.M.; Lee, S. Cytotoxicity of substituted benzimidazolyl curcumin mimics against multi-drug resistance cancer cell. Bull. Korean Chem. Soc. 2013, 34, 1272–1274. [Google Scholar] [CrossRef]
- Hagiwara, H.; Okada, S. A polymorphism-dependent T1/2 shift of 100 K in a hysteretic spin-crossover complex related to differences in intermolecular weak CH⋯X hydrogen bonds (X = S vs. S and N). Chem. Commun. 2016, 52, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Falahati, M.; Irannejad, H.; Emami, S. Synthesis, in vitro antifungal evaluation and in silico study of 3-azolyl-4-chromanone phenylhydrazones. Daru J. Pharm. Sci. 2012, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.H.; Ma, L.; Dai, Z.C.; Xiao, Y.; Zhang, Y.Y.; Li, D.D.; Wang, J.X.; Zhu, H.L. Synthesis and antifungal activity of Nicotinamide derivatives as succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2014, 62, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, H.X.; Li, C.; Wang, J.X.; Li, J.; Wang, M.H.; Ye, Y.H. Antifungal screening of endophytic fungi from Ginkgo biloba for discovery of potent anti-phytopathogenic fungicides. FEMS Microbiol. Lett. 2013, 339, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Wang, H.C.; Wang, J.X.; Chen, C.J.; Zhou, M.G. Fungicidal activity of propiconazole to Rhizoctonia. solani and its control efficacy against rice sheath blight. Plant Prot. 2012, 38, 158–161. [Google Scholar]
- Peng, D.; Li, S.D.; Wang, J.X.; Chen, C.J.; Zhou, M.G. Integrated biological and chemical control of rice sheath blight by Bacillus subtilis NJ-18 and jinggangmycin. Pest Manag. Sci. 2014, 70, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.L.; Zhang, Y.Y.; Liu, Y.J.; Yang, T.T.; Zhang, J.L.; Zhang, Z.G.; Ye, Y.H. Anti-phytopathogenic activity of sporothriolide, a metabolite from endophyte Nodulisporium. sp. A21 in Ginkgo biloba. Pestic. Biochem. Phys. 2016, 129, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Tang, W.; Liu, K.; Huang, Q.; Zhang, X.; Yan, X. Eight RGS and RGS-like proteins orchestrate growth, differentiation, and pathogenicity of Magnaporthe oryzae. PLoS Pathog. 2011, 7, e100245012. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.






| Compound | R1 | R2 | R3 | EC50 (±SD) μg/mL | |
|---|---|---|---|---|---|
| R. solani | M. oryzae | ||||
| 6a | H | H | H | 2.86 ± 0.15 | 11.09 ± 0.92 |
| 6b b | H | H | 2-F | 2.14 ± 0.12 | 6.73 ± 0.56 |
| 6c b | H | H | 3-F | 2.14 ± 0.18 | 9.08 ± 0.63 |
| 6d b | H | H | 4-F | 1.88 ± 0.13 | 8.29 ± 0.28 |
| 6e b | H | H | 4-OCF3 | 1.50 ± 0.08 | 8.22 ± 0.67 |
| 6f b | H | H | 2,4-2F | 1.20 ± 0.10 | 1.85 ± 0.21 |
| 6g b | H | H | 2,5-2F | 3.98 ± 0.19 | 12.39 ± 0.89 |
| 6h b | H | H | 2-Cl | 3.29 ± 0.17 | 5.47 ± 0.49 |
| 6i b | H | H | 3-Cl | 3.77 ± 0.23 | 13.66 ± 0.19 |
| 6j b | H | H | 4-Cl | 1.00 ± 0.06 | 8.71 ± 0.55 |
| 6k b | H | H | 2,4-2Cl | 2.83 ± 0.15 | 9.27 ± 0.81 |
| 6l b | H | H | 2,5-2Cl | 7.47 ± 0.31 | 14.66 ± 1.17 |
| 6m b | H | H | 2,6-2Cl | 5.85 ± 0.22 | 12.87 ± 0.76 |
| 6n b | H | H | 3,5-2Cl | >25 | >25 |
| 6o b | H | H | 2-Br | 4.18 ± 0.21 | 11.33 ± 1.21 |
| 6p b | H | H | 4-Br | 1.14 ± 0.07 | 8.90 ± 1.05 |
| 6q | H | H | 4-OMe | 7.02 ± 0.26 | 16.53 ± 1.24 |
| 6r b | H | H | 4-CN | 7.60 ± 0.23 | 21.18 ± 1.91 |
| 6s b | H | H | 3,4-2CH3 | 6.95 ± 0.29 | 17.62 ± 1.62 |
| 6t | H | CH3 | H | 10.04 ± 0.25 | >25 |
| 6u b | H | CH3 | 3-F | >25 | >25 |
| 6v b | H | CH3 | 2,4-2F | 2.89 ± 0.04 | 7.89 ± 0.31 |
| 6w b | H | CH3 | 2,5-2Cl | >25 | >25 |
| 6x | H | CH3 | 4-Cl | >25 | >25 |
| 6y b | H | CH3 | 2,4-2Cl | >25 | >25 |
| 6z | H | CH3 | 2-Br | >25 | >25 |
| 6aa b | H | CH3 | 4-OCF3 | >25 | >25 |
| 6ab b | H | CH3 | 4-F | 3.01 ± 0.02 | >25 |
| 6ac b | H | CH3 | 4-CN | >25 | >25 |
| 6ad b | CH3 | H | H | 3.84 ± 0.13 | 19.77 ± 0.65 |
| 6ae b | CH3 | H | 4-Cl | 1.28 ± 0.09 | 11.85 ± 0.34 |
| 6af b | CH3 | H | 2,4-2F | 2.30 ± 0.11 | 6.54 ± 0.19 |
| 6ag b | Cl | H | H | 2.65 ± 0.06 | 14.24 ± 0.58 |
| 6ah b | Cl | H | 4-Cl | 0.93 ± 0.04 | 10.40 ± 0.24 |
| 6ai b | Cl | H | 2,4-2F | 1.15 ± 0.09 | 5.26 ± 0.14 |
| carbendazim | 1.84 ± 0.04 | 1.87 ± 0.10 | |||
| validamycin A | 5.07 ± 0.28 | ||||
| isoprothiolane | 0.02 ± 0.01 | ||||
| Compound | Treatment (μg/mL) | ||
|---|---|---|---|
| 1 | 10 | 50 | |
| 6f | 0 | 24.4% | 53.1% |
| validamycin A | 26.3% | 52.7% | 100% |
| Compound | Treatment (μg/mL) | Lesion Lengtha (mm) | Protection Efficacy (%) |
|---|---|---|---|
| 6f | 200 | 5.1 | 68.9 |
| 100 | 5.5 | 66.5 | |
| validamycin A | 200 | 4.3 | 73.8 |
| 100 | 6.4 | 61.0 | |
| blank control | 16.4 |
| Compound | Treatment (μg/mL) | Blast Lesions | Protection Efficacy (%) |
|---|---|---|---|
| 6f | 50 | 32.7 | 21.6 |
| 100 | 26 | 37.6 | |
| carbendazim | 25 | 11.3 | 72.9 |
| 50 | 5.0 | 88.0 | |
| blank control | 41.7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, Y.-F.; Yan, W.; Cao, L.-L.; Ye, Y.-H. Synthesis and Biological Evaluation of Benzimidazole Phenylhydrazone Derivatives as Antifungal Agents against Phytopathogenic Fungi. Molecules 2016, 21, 1574. https://doi.org/10.3390/molecules21111574
Wang X, Chen Y-F, Yan W, Cao L-L, Ye Y-H. Synthesis and Biological Evaluation of Benzimidazole Phenylhydrazone Derivatives as Antifungal Agents against Phytopathogenic Fungi. Molecules. 2016; 21(11):1574. https://doi.org/10.3390/molecules21111574
Chicago/Turabian StyleWang, Xing, Yong-Fei Chen, Wei Yan, Ling-Ling Cao, and Yong-Hao Ye. 2016. "Synthesis and Biological Evaluation of Benzimidazole Phenylhydrazone Derivatives as Antifungal Agents against Phytopathogenic Fungi" Molecules 21, no. 11: 1574. https://doi.org/10.3390/molecules21111574
APA StyleWang, X., Chen, Y. -F., Yan, W., Cao, L. -L., & Ye, Y. -H. (2016). Synthesis and Biological Evaluation of Benzimidazole Phenylhydrazone Derivatives as Antifungal Agents against Phytopathogenic Fungi. Molecules, 21(11), 1574. https://doi.org/10.3390/molecules21111574