A Comparative Study of Enantioseparations of Nα-Fmoc Proteinogenic Amino Acids on Quinine-Based Zwitterionic and Anion Exchanger-Type Chiral Stationary Phases under Hydro-Organic Liquid and Subcritical Fluid Chromatographic Conditions
Abstract
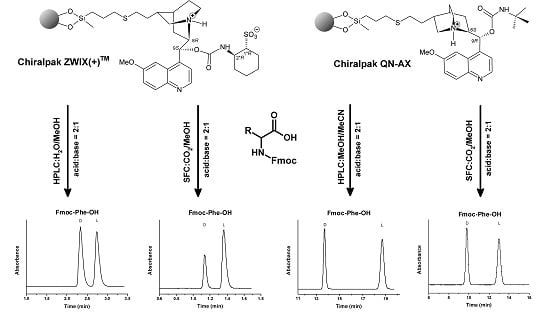
:1. Introduction
2. Results
2.1. Separation of Nα-Fmoc Proteinogenic Amino Acids on Quinine-Based CSPs under Liquid Chromatographic Conditions
2.1.1. Effect of Bulk Solvent Composition
2.1.2. Effect of Base and Acid Additives
2.1.3. Effect of Counter-Ion Content
2.1.4. Comparison of Separation Performances of Zwitterionic and Anion-Exchanger Type CSPs in Liquid Chromatographic Mode
2.2. Separation of Nα-Protected Amino Acids on Quinine-Based CSPs under SFC Conditions
2.2.1. Effect of Co-Solvent and Water Content
2.2.2. Effects of Acid and Base Additives
2.2.3. Effects of the Counter-Ion Concentration
2.2.4. Comparison of Separation Performances of Zwitterionic and Anion-Exchanger Type CSPs Operated in SFC Mode
2.3. Influence of Temperature on the Separation of Nα-Fmoc Amino Acids on Quinine-Based CSPs in HO, PI and SFC Mode
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus and Chromatography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of interest
References
- Machida, Y.; Nishi, H. Chiral Purity in Drug Analysis. In Encyclopedia of Analytical Chemistry; Wiley-VCH: Weinhiem, Germany, 2006. [Google Scholar]
- Whitford, D. Proteins. Structures and functions; John Wiley and Sons: Chichester, UK, 2005. [Google Scholar]
- Dawson, P.E.; Muir, T.W.; Clark-Lewis, I.; Kent, I. Synthesis of proteins by native chemical ligation. Science 1994, 266, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.J. The use of preparative chiral chromatography for accessing enantiopurity in pharmaceutical discovery and development. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Comprehensive Organic Synthesis II, 2nd ed.; Elsevier: Waltham, MA, USA, 2014; Volume 9, pp. 143–159. [Google Scholar]
- Ilisz, I.; Berkecz, R.; Péter, A. Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers: A review. J. Pharm. Biomed. Anal. 2008, 47, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Berkecz, R.; Péter, A. HPLC separation of amino acid enantiomers and small peptides on macrocyclic antibiotic-based chiral stationary phases: A review. J. Sep. Sci. 2006, 29, 1305–1321. [Google Scholar] [CrossRef] [PubMed]
- Toyo’oka, T. Fluorescent chiral derivatization reagents possessing benzofurazan structure for the resolution of optical isomers in HPLC: The synthesis, characteristics and application. Curr. Pharm. Anal. 2005, 1, 57–64. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ikai, T. Chiral HPLC for efficient resolution of enantiomers. Chem. Soc. Rev. 2008, 37, 2593–2608. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Okamoto, Y. Efficient separation of enantiomers using stereoregular chiral polymers. Chem. Rev. 2016, 116, 1094–1138. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Pataj, Z.; Aranyi, A.; Péter, A. High-performance liquid chromatography of biologically important, small epimeric peptides and their l,d-amino acid content. Mini-Rev. Med. Chem. 2010, 10, 287–298. [Google Scholar] [CrossRef] [PubMed]
- B’Hymer, C.; Montes-Bayon, M.; Caruso, J.A. Marfey’s reagent: Past, present, and future uses of 1-fluoro-2,4-dinitrophenyl-5-l-alanine amide. J. Sep. Sci. 2003, 26, 7–19. [Google Scholar] [CrossRef]
- Maier, N.M.; Lindner, W. Chirality in Drug Research, Stereoselective Chromatographic Methods for Drug Analysis; Francotte, E., Lindner, W., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 189–198. [Google Scholar]
- Ilisz, I.; Aranyi, A.; Pataj, Z.; Péter, A. Recent advances in the enantioseparation of amino acids and related compounds, a review. J. Pharm. Biomed. Anal. 2012, 69, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Görög, S. Chromatographic Separations and Analysis: Diastereomeric Derivatization for Chromatography. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Ed.; Elsevier: Waltham, MA, USA, 2016. [Google Scholar]
- West, C.; Lesellier, E. Effects of mobile phase composition on retention and selectivity in achiral supercritical fluid chromatography. J. Chromatogr. A 2013, 1302, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Cazenave-Gassiot, A.; Boughtflower, R.; Caldwell, J.; Hitzel, L.; Holyoak, C.; Lane, S.; Oakley, P.; Pullen, F.; Richardson, S.; Langley, G.J. Effect of increasing concentration of ammonium acetate as an additive in supercritical fluid chromatography using CO2-methanol mobile phase. J. Chromatogr. A 2009, 1216, 6441–6450. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.A. Separation of polar solutes by packed column supercritical fluid chromatography. J. Chromatogr. A 1997, 785, 3–33. [Google Scholar] [CrossRef]
- Francotte, E. Practical advances in SFC for the purification of pharmaceutical molecules. LC-GC Europe 2016, 29, 194–204. [Google Scholar]
- Hoffmann, C.V.; Pell, R.; Lämmerhofer, M.; Lindner, W. Effects on enantioselectivity of zwitterionic chiral stationary phases for separations of chiral acids, bases, and amino acids by HPLC. Anal. Chem. 2008, 80, 8780–8789. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Péter, A.; Lindner, W. State-of-the-art enantioseparations of natural and unnatural amino acids by high-performance liquid chromatography. Trends Anal. Chem. 2016, 81, 11–21. [Google Scholar] [CrossRef]
- Lajkó, G.; Ilisz, I.; Tóth, G.; Fülöp, F.; Lindner, W.; Péter, A. Application of Cinchona alkaloid-based zwitterionic chiral stationary phases in supercritical fluid chromatography for the enantioseparation of Nα-protected proteinogenic amino acids. J. Chromatogr. A 2015, 1415, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Pell, R.; Lindner, W. Potential of chiral anion-exchangers operated in various subcritical fluid chromatography modes for resolution of chiral acids. J. Chromatogr. A 2012, 1245, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wolrab, D.; Kohout, M.; Boras, M.; Lindner, W. Strong cation exchange type chiral stationary phase for enantioseparation of chiral amines in subcritical fluid chromatography. J. Chromatogr. A 2013, 1289, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Wolrab, D.; Macíková, P.; Boras, M.; Kohout, M.; Lindner, W. Strong cation exchange chiral stationary phase–A comparative study in high-performance liquid chromatography and subcritical fluid chromatography. J. Chromatogr. A 2013, 1317, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Allenmark, S.; Schurig, V. Chromatography on chiral stationary phases. J. Mater. Sci. 1977, 7, 1955–1963. [Google Scholar] [CrossRef]
- Spanik, I.; Krupcik, J.; Schurig, V. Comparison of two methods for the gas-chromatographic determination of thermodynamic parameters of enantioselectivity. J. Chromatogr. A 1999, 843, 123–128. [Google Scholar] [CrossRef]
- Fornstedt, T.; Sajonz, P.; Guichon, G. Thermodynamic study of an unusual chiral separation-propanolol enantiomers on an immobilized cellulose. J. Am. Chem. Soc. 1997, 119, 1254–1264. [Google Scholar] [CrossRef]
- Fornstedt, T. Characterization of adsorption processes in analytical liquid chromatography. J. Chromatogr. A 2010, 1217, 792–812. [Google Scholar] [CrossRef] [PubMed]
- Chester, T.L.; Coym, J.W. Effect of phase ratio on van’t Hoff analysis in reversed-phase liquid chromatography, and phase-ratio-independent estimation of transfer enthalpy. J. Chromatogr. A 2003, 1003, 101–111. [Google Scholar] [CrossRef]
- Fornstedt, T.; Sajonz, P.; Guichon, G. A closer study of chiral retention mechanisms. Chirality 1998, 10, 375–381. [Google Scholar] [CrossRef]
- Gotmar, G.; Fornstedt, T.; Guichon, G. Apparent and true enantioselectivity in enantioseparations. Chirality 2000, 12, 558–564. [Google Scholar] [CrossRef]
- Gotmar, G.; Fornstedt, T.; Guichon, G. Retention mechanism of β-blockers on an immobilized cellulase. Relative importance of the hydrophobic and ionic contributions to their enantioselective and nonselective interactions. Anal. Chem. 2000, 72, 3908–3915. [Google Scholar] [CrossRef] [PubMed]
- Péter, A.; Vékes, E.; Armstrong, D.W. Effects of temperature on retention of chiral compounds on a ristocetin—A chiral stationary phase. J. Chromatogr. A 2002, 958, 89–107. [Google Scholar] [CrossRef]
- Cavazzini, G.; Nadalini, G.; Dondi, F.; Gasparrini, F.; Ciogli, A.; Villani, C. Study of mechanisms of chiral discrimination of amino acids and their derivatives on a teicoplanin-based chiral stationary phase. J. Chromatogr. A 2004, 1031, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.V.; Reischl, R.; Maier, N.M.; Lämmerhofer, M.; Lindner, W. Investigations of mobile phase contributions to enantioselective anion- and zwitterion-exchange modes on quinine-based zwitterionic chiral stationary phases. J. Chromatogr. A 2009, 1216, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.V.; Lämmerhofer, M.; Lindner, W. Novel strong cation-exchange type chiral stationary phase for the enantiomer separation of chiral amines by high-performance liquid chromatography. J. Chromatogr. A 2007, 1161, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Baeyens, W.R.G.; Aboul-Enein, H.Y.; Delanghe, J.R.; Tu, T.; Ounyang, J. Impact of amines as co-modifiers on the enantioseparation of various amino acid derivatives on a tert-butyl carbamoylated quinine-based chiral stationary phase. Talanta 2007, 71, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Gecse, Z.; Pataj, Z.; Fülöp, F.; Tóth, G.; Lindner, W.; Péter, A. Direct high-performance liquid chromatographic enantioseparation of secondary amino acids on Cinchona alkaloid-based chiral zwitterionic stationary phases. Unusual temperature behavior. J. Chromatogr. A 2014, 1363, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Gecse, Z.; Lajkó, G.; Forró, E.; Fülöp, F.; Lindner, W.; Péter, A. High-performance liquid chromatographic enantioseparation of cyclic β-amino acids on zwitterionic chiral stationary phases based on Cinchona alkaloids. Chirality 2015, 27, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Grecsó, N.; Papoušek, R.; Pataj, Z.; Barták, P.; Lázár, L.; Fülöp, F.; Lindner, W.; Péter, A. High-performance liquid chromatographic separation of unusual β3-amino acid enantiomers in different chromatographic modes on Cinchona alkaloid-based zwitterionic chiral stationary phases. Amino Acids 2015, 47, 2279–2291. [Google Scholar] [CrossRef] [PubMed]
- Kopaciewicz, W.; Rounds, M.A.; Fausnaugh, F.; Regnier, F.E. Retention model for high performance ion-exchange chromatography. J. Chromatogr. A 1983, 266, 3–21. [Google Scholar] [CrossRef]
- Stahlberg, J. Retention models for ions in chromatography. J. Chromatogr. A 1999, 855, 3–55. [Google Scholar] [CrossRef]
- Péter, A.; Grecsó, N.; Tóth, G.; Fülöp, F.; Lindner, W.; Ilisz, I. Ultra-trace analysis of enantiomeric impurities in proteinogenic N-Fmoc-amino acid samples on Cinchona alkaloid-based chiral stationary phases. Israel J. Chem. 2016. [Google Scholar] [CrossRef]
- Engelhardt, H.; Gross, A.; Mertens, R.; Petersen, M. High-performance liquid-chromatographic columns and stationary phases in supercritical-fluid chromatography. J. Chromatogr. 1989, 477, 169–183. [Google Scholar] [CrossRef]
- Ashraf-Khorassani, M.; Taylor, L.T. Subcritical fluid chromatography of water soluble nucleobases on various polar stationary phases facilitated with alcohol-modified CO2 and water as the polar additive. J. Sep. Sci. 2010, 33, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Pereira, A.; Girón, A.J.; Admasu, E.; Sandra, P. Green hydrophilic interaction chromatography using ethanol-water-carbon dioxide mixtures. J. Sep. Sci. 2010, 33, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Steuer, W.; Schindler, M.; Schill, G.; Emi, F. Supercritical fluid chromatography with ion-pairing modifiers-separation of enantiomeric 1,2-aminoalcohols as diastereomeric ion-pairs. J. Chromatogr. 1988, 447, 287–296. [Google Scholar] [CrossRef]
- Berger, T.A.; Deye, J.F.; Ashraf-Khorassani, M.; Taylor, L.T. Gradient separation of PTH-amino acids employing supercritical CO2 and modifiers. J. Chromatogr. Sci. 1989, 27, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Steuer, W.; Baumann, J.; Emi, F. Separation if ionic drug substances by supercritical fluid chromatography. J. Chromatogr. A 1990, 500, 469–479. [Google Scholar] [CrossRef]
- Biermanns, P.; Miller, C.; Lyon, V.; Wilson, W. Chiral resolution of β-blockers by packed-column supercritical-fluid chromatography. LC-GC 1993, 11, 744–754. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.



 for Asp(OtBu)-OH;
for Asp(OtBu)-OH;  for Fmoc-Lys(Boc)-OH; and
for Fmoc-Lys(Boc)-OH; and  Fmoc-Phe-OH.
Fmoc-Phe-OH.
 for Asp(OtBu)-OH;
for Asp(OtBu)-OH;  for Fmoc-Lys(Boc)-OH; and
for Fmoc-Lys(Boc)-OH; and  Fmoc-Phe-OH.
Fmoc-Phe-OH.



 for Asp(OtBu)-OH;
for Asp(OtBu)-OH;  for Fmoc-Lys(Boc)-OH;
for Fmoc-Lys(Boc)-OH;  for Fmoc-Leu-OH; and
for Fmoc-Leu-OH; and  Fmoc-Phe-OH.
Fmoc-Phe-OH.
 for Asp(OtBu)-OH;
for Asp(OtBu)-OH;  for Fmoc-Lys(Boc)-OH;
for Fmoc-Lys(Boc)-OH;  for Fmoc-Leu-OH; and
for Fmoc-Leu-OH; and  Fmoc-Phe-OH.
Fmoc-Phe-OH.



| Compound | Column | k1 | α | RS | Elution Sequence |
|---|---|---|---|---|---|
| Fmoc-Asp(OtBu)-OH | ZWIX(+)™ | 0.36 | 1.32 | 1.16 | D < L |
| QN-AX™ | 2.38 | 1.82 | 8.77 | D < L | |
| Fmoc-Glu(OtBu)-OH | ZWIX(+)™ | 0.22 | 1.32 | 0.64 | D < L |
| QN-AX™ | 1.97 | 1.88 | 9.54 | D < L | |
| Fmoc-Lys(Boc)-OH | ZWIX(+)™ | 0.19 | 1.43 | 1.01 | D < L |
| QN-AX™ | 1.32 | 1.80 | 6.20 | D < L | |
| Fmoc-Arg(Pbf)-OH | ZWIX(+)™ | 0.99 | 1.91 | 4.67 | D < L |
| QN-AX™ | 2.74 | 1.59 | 7.87 | D < L | |
| Fmoc-His(Trt)-OH | ZWIX(+)™ | 0.63 | 1.66 | 2.89 | D < L |
| QN-AX™ | 1.85 | 1.99 | 10.49 | D < L | |
| Fmoc-Ala-OH | ZWIX(+)™ | 0.25 | 1.36 | 0.88 | D < L |
| QN-AX™ | 2.18 | 1.50 | 6.67 | D < L | |
| Fmoc-Val-OH | ZWIX(+)™ | 0.20 | 1.54 | 0.77 | D < L |
| QN-AX™ | 1.88 | 2.06 | 12.66 | D < L | |
| Fmoc-Leu-OH | ZWIX(+)™ | 0.18 | 1.36 | 0.70 | D < L |
| QN-AX™ | 1.58 | 1.83 | 8.53 | D < L | |
| Fmoc-Ile-OH | ZWIX(+)™ | 0.18 | 1.43 | 0.69 | D < L |
| QN-AX™ | 1.80 | 2.05 | 10.62 | D < L | |
| Fmoc-Phe-OH | ZWIX(+)™ | 0.38 | 1.63 | 2.11 | D < L |
| QN-AX™ | 3.26 | 1.55 | 7.74 | D < L | |
| Fmoc-Trp-OH | ZWIX(+)™ | 0.80 | 2.36 | 6.82 | D < L |
| QN-AX™ | 4.11 | 1.64 | 8.70 | D < L | |
| Fmoc-Met-OH | ZWIX(+)™ | 0.33 | 1.41 | 1.31 | D < L |
| QN-AX™ | 2.84 | 1.67 | 9.83 | D < L | |
| Fmoc-Pro-OH | ZWIX(+)™ | 0.27 | 1.00 | 0.00 | - |
| QN-AX™ | 1.88 | 1.06 | 1.06 | D < L | |
| Fmoc-Ser(tBu)-OH | ZWIX(+)™ | 0.22 | 1.41 | 0.87 | D < L |
| QN-AX™ | 2.14 | 1.35 | 5.42 | D < L | |
| Fmoc-Thr(tBu)-OH | ZWIX(+)™ | 0.19 | 1.00 | 0.00 | - |
| QN-AX™ | 1.72 | 1.05 | 0.52 | - | |
| Fmoc-Cys(Trt)-OH | ZWIX(+)™ | 0.59 | 1.49 | 2.36 | D < L |
| QN-AX™ | 6.77 | 1.14 | 2.68 | D < L | |
| Fmoc-Tyr(tBu)-OH | ZWIX(+)™ | 0.52 | 1.00 | 0.00 | - |
| QN-AX™ | 3.89 | 1.17 | 2.11 | D < L | |
| Fmoc-Asn-OH | ZWIX(+)™ | 0.73 | 2.06 | 5.50 | D < L |
| QN-AX™ | 3.45 | 1.33 | 5.47 | D < L | |
| Fmoc-Gln-OH | ZWIX(+)™ | 0.45 | 1.54 | 2.11 | D < L |
| QN-AX™ | 2.75 | 1.98 | 10.61 | D < L |
| Compound | Column | k1 | α | RS | Elution Sequence |
|---|---|---|---|---|---|
| Fmoc-Asp(OtBu)-OH | ZWIX(+)™ * | 1.76 | 1.18 | 2.43 | D < L |
| QN-AX™ | 5.074 | 1.58 | 8.33 | D < L | |
| Fmoc-Glu(OtBu)-OH | ZWIX(+)™ * | 1.66 | 1.17 | 2.13 | D < L |
| QN-AX™ | 5.01 | 1.64 | 8.86 | D < L | |
| Fmoc-Lys(Boc)-OH | ZWIX(+)™ * | 2.22 | 1.21 | 2.63 | D < L |
| QN-AX™ | 4.781 | 1.51 | 7.11 | D < L | |
| Fmoc-Arg(Pbf)-OH | ZWIX(+)™ * | 24.39 | 1.65 | 9.95 | D < L |
| QN-AX™ | 27.50 | 1.33 | 5.26 | D < L | |
| Fmoc-His(Trt)-OH | ZWIX(+)™ * | 5.19 | 1.31 | 4.36 | D < L |
| QN-AX | 8.20 | 1.47 | 4.64 | D < L | |
| Fmoc-Ala-OH | ZWIX(+)™ * | 2.20 | 1.15 | 2.03 | D < L |
| QN-AX | 5.86 | 1.40 | 6.49 | D < L | |
| Fmoc-Val-OH | ZWIX(+)™ * | 1.60 | 1.24 | 2.79 | D < L |
| QN-AX | 4.46 | 1.71 | 9.87 | D < L | |
| Fmoc-Leu-OH | ZWIX(+)™ * | 1.62 | 1.20 | 2.11 | D < L |
| QN-AX™ | 4.05 | 1.64 | 8.95 | D < L | |
| Fmoc-Ile-OH | ZWIX(+)™ * | 1.42 | 1.21 | 1.49 | D < L |
| QN-AX™ | 4.20 | 1.72 | 9.82 | D < L | |
| Fmoc-Phe-OH | ZWIX(+)™ * | 3.35 | 1.31 | 4.53 | D < L |
| QN-AX™ | 9.887 | 1.36 | 6.04 | D < L | |
| Fmoc-Trp-OH | ZWIX(+)™ * | 16.40 | 2.02 | 10.70 | D < L |
| QN-AX | 26.98 | 1.45 | 7.42 | D < L | |
| Fmoc-Met-OH | ZWIX(+)™ * | 2.95 | 1.23 | 3.41 | D < L |
| QN-AX™ | 8.55 | 1.52 | 8.13 | D < L | |
| Fmoc-Pro-OH | ZWIX(+)™ * | 1.66 | 1.00 | 0.00 | - |
| QN-AX™ | 4.04 | 1.00 | 0.00 | - | |
| Fmoc-Ser(tBu)-OH | ZWIX(+)™ * | 1.26 | 1.13 | 0.96 | D < L |
| QN-AX™ | 3.98 | 1.23 | 3.64 | D < L | |
| Fmoc-Thr(tBu)-OH | ZWIX(+)™ * | 3.67 | 1.95 | 9.42 | - |
| QN-AX™ | 2.73 | 1.17 | 2.70 | D < L | |
| Fmoc-Cys(Trt)-OH | ZWIX(+)™ * | 7.92 | 1.07 | 1.20 | D < L |
| QN-AX™ | 15.84 | 1.73 | 10.49 | D < L | |
| Fmoc-Tyr(tBu)-OH | ZWIX(+)™ * | 2.67 | 1.33 | 4.39 | - |
| QN-AX™ | 10.63 | 1.94 | 12.36 | D < L | |
| Fmoc-Asn-OH | ZWIX(+)™ * | 8.95 | 1.35 | 5.73 | D < L |
| QN-AX™ | 14.54 | 1.23 | 3.94 | D < L | |
| Fmoc-Gln-OH | ZWIX(+)™ * | 6.57 | 1.49 | 7.41 | D < L |
| QN-AX™ | 13.04 | 2.31 | 15.10 | D < L |
| Compound | Column/Mobile Phase | −Δ(ΔH°) (kJ·mol−1) | −Δ(ΔS°) (J·mol−1·K−1) | −TxΔ(ΔS°)298 K (kJ·mol−1) | −Δ(ΔG°)298 K (kJ·mol−1) | Q |
|---|---|---|---|---|---|---|
| Fmoc-Asp(OtBu)-OH | ZWIX(+)™ HO/k | 5.6 | 16.7 | 5.0 | 0.6 | 1.1 |
| QN-AX™PIM/w | 5.5 | 13.5 | 4.0 | 1.5 | 1.4 | |
| ZWIX(+)™ ∗ SFC/y | 0.8 | 1.3 | 0.4 | 0.4 | 2.0 | |
| QN-AX™SFC/x | 3.6 | 7.5 | 2.2 | 1.4 | 1.6 | |
| Fmoc-Lys(Boc)-OH | ZWIX(+)™HO/k | 3.5 | 10.1 | 3.0 | 0.5 | 1.2 |
| QN-AX ™PIM/w | 3.8 | 7.8 | 2.3 | 1.5 | 1.7 | |
| ZWIX(+)™ ∗ SFC/y | 1.4 | 2.7 | 0.8 | 0.6 | 1.8 | |
| QN-AX ™SFC/x | 1.9 | 2.5 | 0.7 | 1.2 | 2.7 | |
| Fmoc-Leu-OH | ZWIX(+)™HO/k | 2.8 | 7.0 | 2.1 | 0.7 | 1.2 |
| QN-AX ™ PIM/w | 4.2 | 9.1 | 2.7 | 1.5 | 1.6 | |
| ZWIX(+)™ ∗ SFC/y | 0.8 | 1.1 | 0.3 | 0.5 | 2.7 | |
| QN-AX ™ SFC/x | 5.1 | 12.1 | 3.6 | 1.5 | 1.4 | |
| Fmoc-Phe-OH | ZWIX(+)™ HO/k | 7.1 | 19.7 | 5.9 | 1.2 | 1.2 |
| QN-AX ™ PIM/w | 4.0 | 9.6 | 2.9 | 1.1 | 1.4 | |
| ZWIX(+)™ ∗ SFC/y | 2.0 | 4.2 | 1.3 | 0.7 | 1.5 | |
| QN-AX ™ SFC/x | 2.1 | 4.3 | 1.3 | 0.8 | 1.6 | |
| Fmoc-Tyr(tBu)-OH | ZWIX(+)™ HO/k | 7.5 | 23.7 | 7.1 | 0.4 | 1.1 |
| QN-AX ™ PIM/w | 2.6 | 7.3 | 2.2 | 0.4 | 1.2 | |
| ZWIX(+)™ ∗ SFC/y | 2.7 | 6.2 | 1.8 | 0.9 | 1.5 | |
| QN-AX ™ SFC/x | 2.4 | 2.4 | 0.7 | 1.7 | 3.4 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajkó, G.; Grecsó, N.; Tóth, G.; Fülöp, F.; Lindner, W.; Péter, A.; Ilisz, I. A Comparative Study of Enantioseparations of Nα-Fmoc Proteinogenic Amino Acids on Quinine-Based Zwitterionic and Anion Exchanger-Type Chiral Stationary Phases under Hydro-Organic Liquid and Subcritical Fluid Chromatographic Conditions. Molecules 2016, 21, 1579. https://doi.org/10.3390/molecules21111579
Lajkó G, Grecsó N, Tóth G, Fülöp F, Lindner W, Péter A, Ilisz I. A Comparative Study of Enantioseparations of Nα-Fmoc Proteinogenic Amino Acids on Quinine-Based Zwitterionic and Anion Exchanger-Type Chiral Stationary Phases under Hydro-Organic Liquid and Subcritical Fluid Chromatographic Conditions. Molecules. 2016; 21(11):1579. https://doi.org/10.3390/molecules21111579
Chicago/Turabian StyleLajkó, Gyula, Nóra Grecsó, Gábor Tóth, Ferenc Fülöp, Wolfgang Lindner, Antal Péter, and István Ilisz. 2016. "A Comparative Study of Enantioseparations of Nα-Fmoc Proteinogenic Amino Acids on Quinine-Based Zwitterionic and Anion Exchanger-Type Chiral Stationary Phases under Hydro-Organic Liquid and Subcritical Fluid Chromatographic Conditions" Molecules 21, no. 11: 1579. https://doi.org/10.3390/molecules21111579
APA StyleLajkó, G., Grecsó, N., Tóth, G., Fülöp, F., Lindner, W., Péter, A., & Ilisz, I. (2016). A Comparative Study of Enantioseparations of Nα-Fmoc Proteinogenic Amino Acids on Quinine-Based Zwitterionic and Anion Exchanger-Type Chiral Stationary Phases under Hydro-Organic Liquid and Subcritical Fluid Chromatographic Conditions. Molecules, 21(11), 1579. https://doi.org/10.3390/molecules21111579