Synthesis, Structure and Cytotoxic Activity of Mono- and Dialkoxy Derivatives of 5,8-Quinolinedione
Abstract
:1. Introduction

2. Results and Discussion
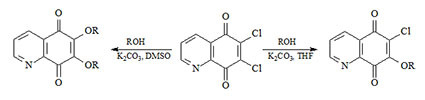
2.1. Chemistry



2.2. Crystal Structure



2.3. Cytotoxic Activity
| Compound | Human Cell Line/IC50 ± SD [µM] | ||||
|---|---|---|---|---|---|
| R | C-32 | SNB-19 | MDA-MB-231 | HFF-1 | |
| 1 | - | 22.8 ± 0.7 | 26.2 ± 0.8 | 25.1 ± 3.1 | 15.7 ± 0.5 |
| 2 | CH3 | 19.2 ± 2.1 | 42.2 ± 0.1 | 1.5 ± 0.2 | 27.6 ± 2.1 |
| 3 | CH2CH3 | 30.7 ± 1.1 | 10.3 ± 2.4 | 24.0 ± 2.8 | 1.3 ± 0.5 |
| 4 | CH2CH2CH3 | 0.20 ± 0.01 | 0.36 ± 0.03 | 22.4 ± 2.7 | Neg |
| 5 | CH2CH =CH2 | 0.28 ± 0.09 | 0.30 ± 0.01 | 17.2 ± 2.8 | Neg |
| 6 | CH2CH2Cl | 22.8 ± 2.5 | 18.3 ± 1.4 | 17.0 ± 3.0 | 15.8 ± 1.2 |
| 7 | CH2CH2CN | 119.4 ± 4.3 | 58.3 ± 2.8 | 164.5 ± 3.9 | 2.5 ± 0.4 |
| 8 |  | 1.4 ± 0.1 | 1.8 ± 0.1 | 3.5 ± 0.3 | 13.9 ± 3.0 |
| 9 |  | 0.81 ± 0.01 | 2.3 ± 0.5 | 271.3 ± 9.8 | 2.0 ± 0.3 |
| 10 | CH3 | 21.5 ± 2.0 | 3.5 ± 0.7 | 3.1 ± 0.1 | 3.1 ± 0.8 |
| 11 | CH2CH3 | 17.6 ± 1.6 | 2.1 ± 0.1 | 18.6 ± 2.0 | Neg |
| 12 | CH2CH2CH3 | 1.1 ± 0.1 | 0.7 ± 0.02 | 3.0 ± 0.3 | Neg |
| 13 | CH2CH =CH2 | 1.5 ± 0.01 | 0.2 ± 0.01 | 12.6 ± 2.7 | Neg |
| 14 | CH2CH2Cl | 12.1 ± 0.2 | 3.5 ± 0.3 | 10.9 ± 1.4 | 1.9 ± 0.2 |
| 15 | CH2CH3 | 10.9 ± 1.3 | 14.9 ± 0.7 | 2.7 ± 0.1 | 12.4 ± 1.2 |
| 16 | CH2CH2CN | 134.4 ± 10.7 | 203.7 ± 14.3 | 110.7 ± 3.7 | 18.2 ± 2.6 |
| 17 |  | 1.4 ± 0.1 | 1.7 ± 0.3 | 13.8 ± 0.1 | Neg |
| 18 |  | 18.7 ± 0.9 | 137.4 ± 0.7 | 107.9 ± 10.5 | 17.2 ± 1.7 |
| cisplatin | 16.4 ± 1.2 | 18.9 ± 0.4 | 25.5 ± 0.2 | 9.1 ± 1.8 | |
3. Materials and Methods
3.1. General Techniques
3.2. General Procedure for the Synthesis of 6-Chloro-7-alkoxy-5,8-quinolinedione
3.3. General Procedure for the Synthesis of 6,7-Dialkoxy-5,8-quinolinedione
3.4. Crystal Structure Determination
Refinement
3.5. Biological Study
3.5.1. Cell Culture
3.5.2. Analysis of the Biological Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Health Estimates Summary. Available online: http://www.who.int (accessed on 1 June 2013).
- Rao, K.V.; Cullen, W.P. Streptonigrin, an antitumor substance. I. Isolation and characterization. Antibiot. Annu. 1960, 7, 950–953. [Google Scholar]
- Boger, D.L.; Yasuda, M.; Mitscher, L.A.; Drake, S.D.; Kitos, P.A.; Thompson, S.C. Streptonigrin and lavendamycin partial structures. Probes for the minimum, potent pharmacophore of streptonigrin, lavendamycin, and synthetic quinoline-5,8-diones. J. Med. Chem. 1987, 30, 1913–1928. [Google Scholar] [CrossRef]
- Bringmann, G.; Reichert, Y.; Kane, V.V. The total synthesis of streptonigrin and related antitumor antibiotic natural products. Tetrahedron 2004, 60, 3539–3574. [Google Scholar] [CrossRef]
- Bringmann, G.; Reichert, M.; Hemnberger, J. The absolute configuration of streptonigrin. Tetrahedron 2008, 64, 515–528. [Google Scholar] [CrossRef]
- Donohoe, T.J.; Jones, C.R.; Kornahrens, A.F.; Barbosa, L.C.; Walport, L.J.; Tatton, M.T.; O’Hagan, M.; Rathi, A.H.; Baker, B.D. Total synthesis of the antitumor antibiotic (±)-streptonigrin: First- and second-generation routes for de novo pyridine formation using ring-closing metathesis. J. Org. Chem. 2013, 78, 12338–12350. [Google Scholar] [CrossRef] [PubMed]
- Fryatt, T.; Goroski, D.T.; Nilson, Z.D.; Moody, C.J.; Beall, H. Novel quinolinequinone antitumor agents: Structure-metabolism studies with NAD(P)H:quinone oxidoreductase (NQO1). Bioorg. Med. Chem. Lett. 1999, 9, 2195–2198. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Jeong, H.J.; Lee, S.K.; You, H.-J.; Chio, K.U.; Shim, J.-Y.; Heo, Y.H.; Lee, C.-O. Effects of 6-arylamine-5,8-quinolinediones and 6-chloro 7-arylo-5,8-isoquinolinediones on NAD(P)H: Quinone oxidoreductase (NQO1) activity and their cytotoxic potential. Arch. Pharm. Res. 2001, 24, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.K.; Park, H.J.; Lee, S.K.; Lee, C.-O.; Choo, H.-Y. Synthesis, cytotoxicity, and DNA topoisomerase II inhibitory activity of benzofuroquinolinediones. Bioorg. Med. Chem. 2007, 15, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Leteurtre, F.; Kohlhagen, G.; Pommier, Y. Streptonigrin induced topoisomerase II sites exhibit base preferences in the middle of the enzym stragger. Biochem. Biophys. Pres. Commun. 1994, 203, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Park, S.-Y.; Lee, H.-J.; Suh, M.-E.; Schollmeyer, D.; Lee, C.-O. Synthesis and cytotoxicity of 6,11-dihydro-pyrido- and 6,11-dihydro-benzo[2,3-b]phenazine-6,11-dione derivatives. Bioorg. Med. Chem. 2003, 11, 1709–1714. [Google Scholar] [CrossRef]
- Shaikh, I.A.; Johnson, F.; Grollman, A.P. Streptonigrin. 1. Structure-activity relationships among simple bicyclic analogs. Rate dependence of DNA degradation on quinone reduction potential. J. Med. Chem. 1986, 29, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Hassani, M.; Karki, R.; Walter, E.D.; Koelsch, K.K.; Seradj, H.; Lineswala, J.P.; Mirzaei, H.; York, Y.S.; Olang, F.; et al. Synthesis, metabolism and in vitro cytotoxicity studies on novel lavendamycin antitumor agents. Bioorg. Med. Chem. 2010, 18, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; Cai, W.; Holley, D.C.; Lineswala, J.P.; Maharjan, B.R.; Ebrahimian, G.R.; Seradj, H.; Stocksdale, M.G.; Mohammadi, F.; Marvin, C.C; et al. Novel lavendamycin analogues as antitumor agents: Aynthesis, in vitro cytotoxicity, structure-metabolism, and computational molecular modeling studies with NAD(P)H: Quinone oxidoreductase 1. J. Med. Chem. 2005, 48, 7733–7749. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Ko, J.H.; Lee, K.I. Cesium carbonate-mediated reaction of dichloronaphthoquinone derivatives with O-nucleophiles. Monatsh. Chem. 2007, 138, 741–746. [Google Scholar] [CrossRef]
- Mulchin, B.J.; Newton, C.G.; Baty, J.W.; Grasso, C.H.; Martin, W.J.; Walton, M.C.; Dangerfield, E.M.; Plunkett, C.H.; Berridge, M.V.; Harper, J.L; et al. The anti-cancer, anti-inflammatory and tuberculostatic activities of a series of 6,7-substituted-5,8-quinolinequinones. Bioorg. Med. Chem. 2010, 18, 3238–3251. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, M.; Boryczka, S.; Kadela, M.; Wrzalik, R.; Kusz, J.; Nowak, M. Synthesis, crystal structure and infrared spectra of new 6- and 7-propylamine-5,8-quinolinediones. J. Mol. Struct. 2014, 1067, 160–168. [Google Scholar] [CrossRef]
- Yoon, E.Y.; Choi, H.Y.; Shin, K.J.; Yoo, K.H.; Chi, D.Y.; Kim, D.J. The regioselectivity in the reaction of 6,7-dihaloquinoline-5,8-diones with amine nucleophiles in various solvents. Tetrahedron Lett. 2000, 41, 7475–7480. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Sun, Y.-J.; Shim, J.-Y.; You, H.-J.; Choi, K.U.; Lee, H. Synthesis and antyfungal activity of 6,7-bis-[S-(aryl)tio]-5,8-quinolinediones. Arch. Pharm. Res. 2002, 25, 795–800. [Google Scholar]
- Bhuiyan, M.M.H.; Ferdaush, J.; Uddin, M.H. Densities and viscosities of binary mixtures of {dimethylsulfoxide + aliphatic lower alkanols (C1–C3)} at temperatures from T = 303.15 K to T = 323.15 K. J. Chem. Thermodyn. 2007, 39, 675–683. [Google Scholar] [CrossRef]
- Kiefer, J.; Noack, K.; Kirchner, B. Hydrogen bond in mixtures of dimethyl sulfoxide and cosolvents. Curr. Phys. Chem. 2011, 1, 340–351. [Google Scholar] [CrossRef]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. 2002, 41, 48–79. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadela, M.; Jastrzębska, M.; Bębenek, E.; Chrobak, E.; Latocha, M.; Kusz, J.; Książek, M.; Boryczka, S. Synthesis, Structure and Cytotoxic Activity of Mono- and Dialkoxy Derivatives of 5,8-Quinolinedione. Molecules 2016, 21, 156. https://doi.org/10.3390/molecules21020156
Kadela M, Jastrzębska M, Bębenek E, Chrobak E, Latocha M, Kusz J, Książek M, Boryczka S. Synthesis, Structure and Cytotoxic Activity of Mono- and Dialkoxy Derivatives of 5,8-Quinolinedione. Molecules. 2016; 21(2):156. https://doi.org/10.3390/molecules21020156
Chicago/Turabian StyleKadela, Monika, Maria Jastrzębska, Ewa Bębenek, Elwira Chrobak, Małgorzata Latocha, Joachim Kusz, Maria Książek, and Stanisław Boryczka. 2016. "Synthesis, Structure and Cytotoxic Activity of Mono- and Dialkoxy Derivatives of 5,8-Quinolinedione" Molecules 21, no. 2: 156. https://doi.org/10.3390/molecules21020156
APA StyleKadela, M., Jastrzębska, M., Bębenek, E., Chrobak, E., Latocha, M., Kusz, J., Książek, M., & Boryczka, S. (2016). Synthesis, Structure and Cytotoxic Activity of Mono- and Dialkoxy Derivatives of 5,8-Quinolinedione. Molecules, 21(2), 156. https://doi.org/10.3390/molecules21020156