Development of Wax-Incorporated Emulsion Gel Beads for the Encapsulation and Intragastric Floating Delivery of the Active Antioxidant from Tamarindus indica L.
Abstract
:1. Introduction
2. Results and Discussion
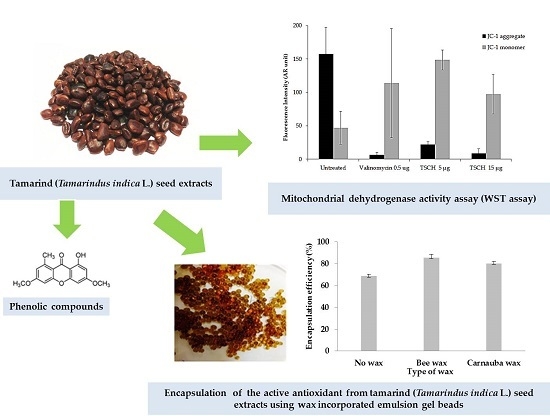
2.1. Tamarind Seed Extracts
2.2. Total Phenolic Content of Extracts
2.3. Superoxide Anion (O2•−) Scavenging Activity of Extracts
2.4. Mitochondrial Dehydrogenase Activity Assay (WST Assay) of Extracts
2.5. Mitochondrial Membrane Potential (MMP Assay) of Extracts
2.6. Conventional Gel Beads and Wax-Incorporated Emulsion Gel Beads
2.7. Particle Size of Gel Beads
2.8. Percentage of Encapsulation Efficiency (% EE)
2.9. Percentage of Active Ingredient Release
3. Materials and Methods
3.1. Materials
3.2. Extraction Conditions
3.3. Determination of Total Phenolic Content
3.4. Determination of Superoxide Anion (O2•−) Scavenging Activity
3.5. Determination of Mitochondrial Toxicity
3.5.1. Cell Culture and Reagents
3.5.2. Mitochondrial Dehydrogenase Activity Assay (WST Assay)
3.5.3. Mitochondrial Membrane Potential Assay (MMP Assay)
3.6. Preparation of Conventional Calcium Alginate Gel Beads and Wax-Incorporated Alginate-Based Emulsion Gel Beads
3.6.1. Preparation of Conventional Calcium Alginate Gel Beads
3.6.2. Wax-Incorporated Alginate-Based Emulsion Gel Beads
3.7. Study of the Particle Size of the Gel Beads
3.8. Determination of Percentage of Encapsulation Efficiency (% EE)
3.9. Determination of Active Ingredient Release
3.10. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sriamornsak, P.; Thirawong, N.; Puttipipatkhachorn, S. Emulsion gel beads of calcium pectinate capable of floating on the gastric fluid: Effect of some additives, hardening agent or coating on release behavior of metronidazole. Eur. J. Pharm. Sci. 2005, 24, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, N.S.; Bharathi, G.; Jayanthi, C.; Hanumanthachar, J. Comparative evaluation of wax incorporated alginate and pectinate gel beads of Metformin. Der Pharm. Lett. 2014, 6, 10–16. [Google Scholar]
- Miharaa, Y.; Sikder, T.; Yamagishi, H.; Sasaki, T.; Kurasaki, M.; Itoha, S.; Tanaka, S. Adsorption kinetic model of alginate gel beads synthesized micro particle-prussian blue to remove cesium ions from water. J. Water Proc. Eng. 2016, 10, 9–119. [Google Scholar] [CrossRef]
- Cataldo, S.; Muratore, N.; Orecchio, S.; Pettignano, A. Enhancement of adsorption ability of calcium alginate gel beads towards Pd(II) ion. A kinetic and equilibrium study on hybrid Laponite and Montmorillonite–alginate gel beads. Appl. Clay Sci. 2015, 118, 162–170. [Google Scholar] [CrossRef]
- Dalatya, A.A.; Karama, A.; Najlahc, M.; Alanyd, R.G.; Khodera, M. Effect of non-cross-linked calcium on characteristics, swelling behaviour, drug release and mucoadhesiveness of calcium alginatebeads. Carbohydr. Polym. 2016, 140, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Shaik, A.A.; Khair, U.; Praneetha, P. Preparation and in vitro evaluation of Chitosan-Carrageenan, Chitosan-Alginate beads for controlled release of Nateglinide. Der Pharm. Sin. 2011, 2, 375–384. [Google Scholar]
- Sriamornsak, P.; Asavapichayont, P.; Nunthanid, J.; Luangtana-anan, M.; Limmatvapirat, S.; Piriyaprasarth, S. Wax-incorporated emulsion gel beads of calcium pectinate for intragastric floating Drug Delivery. AAPS PharmSciTech 2008, 9, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calciumnanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Tateshita, K.; Sugawara, S.; Imai, T.; Otagiri, M. Preparation and evaluationof a controlled-release formulation of nifedipine using alginate gel beads. Biol. Pharm. Bull. 1993, 16, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P.; Nunthanid, J. Calcium pectinate gel beads for controlled release drug delivery: I. Preparation and In-vitro release studies. Int. J. Pharm. 1998, 160, 207–212. [Google Scholar] [CrossRef]
- Gavini, V.; Reddy, B.P.; Konijeti, S.R.; Kumar, K. Formulation & In-Vitro Evaluation of Wax Incorporated Floating Beads of SilymarinInt. J. PharmTech Res. 2014, 6, 1824–1832. [Google Scholar]
- Siddhuraju, P. Antioxidant activity of polyphenolic compounds extracted from defatted raw and dry heated Tamarindus indica seed coat. LWT-Food Sci. Technol. 2007, 40, 982–990. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bal, S.; Mukherjee, R.K.; Bhattacharya, S. Functional and nutritional properties of tamarind (Tamarindus indica) kernel protein. Food Chem. 1994, 49, 1–9. [Google Scholar] [CrossRef]
- Tsuda, T.; Watanabe, M.; Ohshima, K. Antioxidative components isolated from the seed of tamarind (Tamarindus indica L.). J. Agric. Food Chem. 1994, 42, 2671–2674. [Google Scholar] [CrossRef]
- Doughari, J.H. Antimicrobial Activity of Tamarindus indica Linn. Trop. J. Pharm. Res. 2006, 5, 597–603. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Obiiyeke, G.E.; Chigor, V.N.; Okoh, A.I. Assessment of Tamarindus indica Extracts for Antibacterial Activity. Int. J. Mol. Sci. 2011, 12, 6385–6396. [Google Scholar] [CrossRef] [PubMed]
- Paula, F.S.; Kabeya, L.M.; Kanashiro, A. Modulation of human neutrophil oxidative metabolism and degranulation by extract of Tamarindus indica L. fruit pulp. Food Chem. Toxicol. 2009, 47, 163–170. [Google Scholar] [CrossRef] [PubMed]
- De, M.; De Krishna, A.; Baneerjee, A.B. Antimicrobial screening of some Indian spices. Phytother. Res. 1999, 13, 616–618. [Google Scholar] [CrossRef]
- Maiti, R.; Das, U.K.; Ghosh, D. Attenuation of hyperglycemia and hyperlipidemia in streptozotocin induced diabetic rats by aqueous extract of seed of Tamarindus indica. Biol. Pharm. Bull. 2005, 28, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.; Jana, D.; Das, U.K. Antidiabetic effect of aqueous extract of seed of Tamarindus indica in strptozotocin-induced diabetic rats. J. Ethnopharmacol. 2004, 92, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Luengthanaphol, S.; Mongkholkhajornsilp, D.; Douglas, S.; Douglas, P.L.; Pengsopa, L.; Pongamphai, S. Extraction of antioxidant from sweet Thai tamarind seed coat-preliminary experiments. J. Food Eng. 2004, 63, 247–252. [Google Scholar] [CrossRef]
- De, M.; Krishna, D.A.; Baneerjee, A.B. Antimicrobial screening of some Indian spices. Phytother. Res. 1999, 3, 616–618. [Google Scholar] [CrossRef]
- Nakchat, O.; Meksuriyen, D.; Pongsamart, S. Antioxidant and anti-lipid peroxidation activities of Tamarindus indica seed coat in human fibroblast cells. Indian J. Exp. Biol. 2014, 52, 125–132. [Google Scholar] [PubMed]
- Kulkarni, A.P.; Aradhya, S.M.; Divakar, S. Isolation and identification of a radical scavenging antioxidant-punicalagin from pith and carpellary membrane of pomegranate fruit. Food Chem. 2004, 87, 551–557. [Google Scholar] [CrossRef]
- Aravind, S.R.; Joseph, M.M.; Varghese, S.; Balaram, P. Antitumor and Immunopotentiating Activity of Polysaccharide PST001 Isolated from the Seed Kernel of Tamarindus indica: An In Vivo Study in Mice. Sci. World J. 2012, 361382. [Google Scholar]
- Rodriguez, L.; Passerinim, N.; Cavallari, C.; Cini, M.; Sancin, P.; Fini, A. Description and preliminary evaluation of a new ultrasonic atomizer for spray-congealing processes. Int. J. Pharm. 1999, 183, 133–143. [Google Scholar] [CrossRef]
- Gopalakrishna, P.; Ramarao, B.; Chiranjeevi1, G.; Ramyasri, S. In-Vitro Evaluation of Gastro Retentive Floating Drug Delivery System for Nitrendipine Bilayer Tablets. Int. J. Pharm. Chem. Res. I 2016, 2, 63–72. [Google Scholar]
- Patila, H.; Tiwaria, R.V.; Repkaa, M.A. Recent advancements in mucoadhesive floating drug delivery systems: A mini-review. J. Drug Deliv. Sci. Technol. 2016, 31, 65–71. [Google Scholar] [CrossRef]
- Narang, N. Update Review on: Floating Drug Delivery System (FDDS). Int. J. Appl. Pharm. 2011, 3, 1–7. Available online: http://ijaponline.org/Vol3Issue1/129.pdf (accessed on 29 February 2016). [Google Scholar]
- Cao, Q.R.; Kim, T.W.; Lee, B.J. Photoimages and the release characteristics of lipophilic matrix tablets containing highly water-soluble potassium citrate with high drug loadings. Int. J. Pharm. 2007, 339, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the phenolic compounds are available from the authors.




| Sample | Total Phenolic Content (GAE mg/g Extract) | Inhibition (%) | IC50 (µg/mL) | |||
|---|---|---|---|---|---|---|
| 1000 (µg/mL) | 500 (µg/mL) | 50 (µg/mL) | 5 (µg/mL) | |||
| TSC50 | 23.69 ± 0.29 | 90.45 ± 4.87 | 76.41 ± 0.84 | 61.08 ± 1.42 | 44.10 ± 2.76 | 11.1 ± 0.09 |
| TSC95 | 43.98 ± 0.33 | 94.98 ± 0.82 | 93.58 ± 3.06 | 75.83 ± 1.47 | 54.95 ± 0.64 | <5 |
| TSCH | 39.39 ± 0.19 | 92.44 ± 1.61 | 90.67 ± 0.92 | 77.84 ± 2.22 | 72.49 ± 0.98 | <5 |
| IC50 (µg/mL) | Cancer Cells | Normal Cells | |||
|---|---|---|---|---|---|
| HepG2 | HeLa | Caco2 | L929 | HK-2 | |
| TSC50 | >1000 | >1000 | 602.50 ± 130.25 | >1000 | >1000 |
| TSC95 | >1000 | >1000 | 249.21 ± 49.70 | >1000 | >1000 |
| TSCH | 75.27 ± 10.54 | 15.06 ± 2.54 | 100.73 ± 11.84 | 444.16 ± 45.56 | >1000 |
| Gel Beads with Different Types and Concentrations of Waxes | Mean Diameter (mm) | Total Phenolic Contents (GAE mg/g Extract) |
|---|---|---|
| Gel bead (No wax) | 2.47 ± 0.16 | 27.01 ± 0.72 |
| Gel bead + Bee wax 1% | 2.26 ± 0.08 | 33.65 ± 1.20 |
| Gel bead + Bee wax 2% | 2.23 ± 0.09 | 35.23 ± 2.24 |
| Gel bead + Bee wax 3% | 2.20 ± 0.12 | 36.26 ± 2.37 |
| Gel bead + Carnauba wax 1% | 2.37 ± 0.05 | 31.56 ± 0.74 |
| Gel bead + Carnauba wax 2% | 2.33 ± 0.07 | 32.57 ± 1.29 |
| Gel bead + Carnauba wax 3% | 2.31 ± 0.16 | 33.61 ± 1.19 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soradech, S.; Petchtubtim, I.; Thongdon-A, J.; Muangman, T. Development of Wax-Incorporated Emulsion Gel Beads for the Encapsulation and Intragastric Floating Delivery of the Active Antioxidant from Tamarindus indica L. Molecules 2016, 21, 380. https://doi.org/10.3390/molecules21030380
Soradech S, Petchtubtim I, Thongdon-A J, Muangman T. Development of Wax-Incorporated Emulsion Gel Beads for the Encapsulation and Intragastric Floating Delivery of the Active Antioxidant from Tamarindus indica L. Molecules. 2016; 21(3):380. https://doi.org/10.3390/molecules21030380
Chicago/Turabian StyleSoradech, Sitthiphong, Intira Petchtubtim, Jeerayu Thongdon-A, and Thanchanok Muangman. 2016. "Development of Wax-Incorporated Emulsion Gel Beads for the Encapsulation and Intragastric Floating Delivery of the Active Antioxidant from Tamarindus indica L." Molecules 21, no. 3: 380. https://doi.org/10.3390/molecules21030380
APA StyleSoradech, S., Petchtubtim, I., Thongdon-A, J., & Muangman, T. (2016). Development of Wax-Incorporated Emulsion Gel Beads for the Encapsulation and Intragastric Floating Delivery of the Active Antioxidant from Tamarindus indica L. Molecules, 21(3), 380. https://doi.org/10.3390/molecules21030380