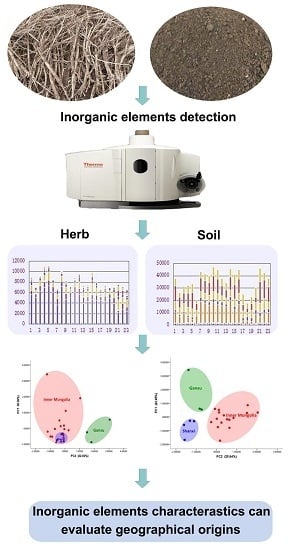
Distinguishing Astragalus mongholicus and Its Planting Soil Samples from Different Regions by ICP-AES
Abstract
:1. Introduction
2. Results and Discussion
2.1. Internal Quality Controls of ICP-AES Procedure
2.1.1. Detecting Wavelength and Detection Limit
2.1.2. Calibration Studies
2.1.3. Accuracy and Precision of the Method
2.2. Inorganic Elements in the Soil
2.3. PCA for Inorganic Elements in Soil of A. mongholicus
2.4. Contents of Inorganic Elements in A. mongholicus
2.5. PCA for Inorganic Elements in A. mongholicus
2.6. Uptake and Accumulation Behavior
2.7. Correlation Analysis
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Sample Preparation
3.3. Calibration Procedure
3.4. Spiking Procedures
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, Z.; Guo, P.; Brand, E. The formation of daodi medicinal materials. J. Ethnopharmacol. 2012, 140, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 8th ed.; Chemical Industry Press: Beijing, China, 2010; pp. 283–284. (In Chinese) [Google Scholar]
- Hong, M.J.; Ko, E.B.; Park, S.K.; Chang, M.S. Inhibitory effect of Astragalus membranaceus root on matrix metalloproteinase-1 collagenase expression and procollagen destruction in ultraviolet B-irradiated human dermal fibroblasts by suppressing nuclear factor kappa-B activity. J. Pharm. Pharmacol. 2013, 65, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.H.; Wang, R.R.; Tian, R.R. Calycosin 7-O-β-d-glucopyranoside, an anti-HIV agent from the roots of Astragalus membranaceus var. mongholicus. Chem. Nat. Compd. 2009, 45, 282–285. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Khanif, Y.M.; Saleem, M. Role of zinc in plant nutrition—A review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar]
- Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar]
- Abou-Arab, A.A.K.; Donia, M.A.A. Heavy metals in Egyptian spices and medicinal plants and the effect of processing on their levels. J. Agric. Food Chem. 2000, 48, 2300–2304. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.H.; Guo, L.P.; Huang, L.Q.; Jin, H. Effects of mineral nutrition on metabolism of flavonoids in medicinal plants. Chin. J. Chin. Mater. Med. 2010, 35, 2367–2371. [Google Scholar]
- Zeng, Y.; Guo, L.P.; Yang, G.; Chen, B.D.; Wang, J.Y.; Huang, L.Q. Effect of environmental ecological factors on Saponins of medicinal plant. Chin. J. Exp. Tradit. Med. Form. 2012, 17, 314–318. [Google Scholar]
- Zhang, L.; Ye, Z.; Guo, Q. Effects of soil factor on active components of Radix Ophiopogonis. Chin. Mater. Med. 2010, 35, 1372–1377. [Google Scholar]
- Chen, Y.H.; Yu, M.M.; Zhu, Z.B. Optimisation of Potassium Chloride Nutrition for Proper Growth, Physiological Development and Bioactive Component Production in Prunella vulgaris L. PLoS ONE 2013, 8, e66259. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.F.; Wu, S.G.; Wang, Y. Soil nutrient factors related to salidroside production of Rhodiola sachalinensis distributed in Chang Bai Mountain. Environ. Exp. Bot. 2004, 52, 267–276. [Google Scholar] [CrossRef]
- Khajeh, M.; Moghaddam, A.R.A.; Sanchooli, E. Application of Doehlert Design in the Optimization of Microwave-Assisted Extraction for Determination of Zinc and Copper in Cereal Samples Using FAAS. Food Anal. Methods 2010, 3, 133–137. [Google Scholar] [CrossRef]
- Tolalioglu, S. Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem. 2012, 134, 2504–2508. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Walter, N.L.; Dannuza, D.C.; Samuel, M.M. Slurry Sampling and HG AFS for the Determination of Total Arsenic in Rice Samples. Food Anal. Methods 2013, 6, 1128–1132. [Google Scholar] [CrossRef]
- Cindric, I.J.; Krizman, I.; Zeiner, M.; Kampic, S.; Medunic, G. ICP-AES determination of minor- and major elements in apples after microwave assisted digestion. Food Chem. 2012, 135, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Wang, T.B.; Antonucci, V. High-efficiency sample preparation with dimethylformamide for multi-element determination in pharmaceutical materials by ICP-AES. J. Pharm. Biomed. 2010, 52, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.G.; Zhao, Y.; Xu, M.S. Simultaneous Determination of 20 Inorganic Elements in Preserved Egg Prepared with Different Metal Ions by ICP-AES. Food Anal. Methods 2013, 6, 667–676. [Google Scholar] [CrossRef]
- Toyama, T. Analysis of metal elements of hydrangea sepals at various growing stages by ICP-AES. Biochem. Eng. J. 2003, 14, 237–241. [Google Scholar] [CrossRef]
- Fu, J.; Yang, S.H.; Huang, L.F. Simultaneous Determination of Six Flavonoid Active Components in Radix Astragali by UPLC. Chin. Pharm. J. 2013, 11, 916–919. [Google Scholar]
- Yang, Q.Z.; Liu, D.W.; Wang, D.M. Correlation on quality and ecotype of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao. Chin. Tradit. Herb. Drugs 2014, 16, 2395–2399. [Google Scholar]
- Fu, J.; Huang, L.F.; Zhang, H.T. Structural features of a polysaccharide from Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao. J. Asian Nat. Prod. Res. 2013, 15, 687–692. [Google Scholar] [CrossRef] [PubMed]
- WHO/FAO/IAEA. Trace Elements in Human Nutrition and Health; World Health Organization: Switzerland, Geneva, 1996. [Google Scholar]
- Yan, L.N. Study on the Effective Components of Atractylodes Plants and Atractylodes macrocephala & Establishment of Herbs’ Element Fingerprint. Master’s thesis, Hunan Normal University, Hunan, China, 30 May 2008. [Google Scholar]
- Christine, S.A.; Mattusch, J.; Reisser, W.; Wennrich, R. Uptake and accumulation behaviour of angiosperms irrigated with solutions of different arsenic species. Chemosphere 2004, 56, 305–313. [Google Scholar]
- Kaushik, A.; Kansal, A.; Santosh; Meena; Kumari, S.; Kaushik, C.P. Heavy metal contamination of river Yamuna, Haryana, India: Assessment by metal enrichment factor of the sediments. J. Hazard. Mater. 2009, 164, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.






| Element | Wavelength (nm) | Regression Equation | LOD, mg/L | LOQ, mg/L | Average Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Pb | 220.54 | y = 171.5763x + 2.0716 | 0.24 | 0.72 | 109.7 | 3.5 |
| Cd | 228.50 | y = 2712.5644x + 6.1782 | 0.04 | 0.12 | 93.7 | 5.5 |
| As | 193.69 | y = 159.8310x − 1.5560 | 0.01 | 0.03 | 108.7 | 6.5 |
| Cu | 324.75 | y = 4410.2400x − 4.4859 | 0.07 | 0.21 | 95.3 | 5.8 |
| P | 213.62 | y = 16.8398x − 0.0549 | 0.11 | 0.33 | 96.7 | 1.6 |
| K | 766.49 | y = 3246.1758x − 114.7232 | 0.08 | 0.24 | 113.3 | 3.3 |
| Zn | 213.85 | y = 2226.9064 + 6.2482 | 0.08 | 0.24 | 104.3 | 2.9 |
| Mn | 257.61 | y = 14,069.62x + 4.9874 | 0.03 | 0.06 | 91.7 | 5.6 |
| Ca | 317.93 | y = 5090.8325x + 15.5628 | 0.14 | 0.42 | 89.0 | 4.5 |
| Mg | 279.50 | y = 100,774.53x + 14.3562 | 0.17 | 0.51 | 88.3 | 3.5 |
| Fe | 259.93 | y = 2422.3150x − 0.5618 | 0.06 | 0.18 | 100.3 | 8.1 |
| Se | 196.02 | y = 92.5530x + 0.9983 | 0.20 | 0.60 | 100.7 | 3.0 |
| B | 249.67 | y = 3074.1732x + 12.5746 | 0.02 | 0.06 | 93.3 | 3.3 |
| Al | 308.21 | y = 1544.3306x − 17.5037 | 0.04 | 0.12 | 106.7 | 0.5 |
| Na | 589.59 | y = 1605.8634x − 313.4292 | 0.09 | 0.27 | 93.3 | 1.6 |
| Cr | 267.71 | y = 5825.6772x + 8.5930 | 0.08 | 0.24 | 85.0 | 4.7 |
| Ni | 231.60 | y = 1401.1639x − 0.0311 | 0.06 | 0.18 | 113.7 | 4.2 |
| Ba | 455.40 | y = 61,217.94x + 202.9208 | 0.48 | 1.44 | 111.0 | 2.4 |
| Hg | 194.16 | y = 475.7408x + 2.5548 | 0.06 | 0.18 | 98.0 | 6.4 |
| Ti | 334.94 | y = 1892.2594x + 15.4562 | 0.09 | 0.27 | 94.0 | 3.8 |
| Sr | 407.77 | y = 3059.3525x + 5.526 | 0.11 | 0.33 | 114.0 | 4.0 |
| Parameters | Value |
|---|---|
| Output power | 1.2 kw |
| Auxiliary air flow | 0.2 L/min |
| Atomization gas flow | 0.8 L/min |
| Cooling flow | 16 L/min |
| The observing pattern | Bidirection |
| The flux of the elevation solution | 1.5 mL/min |
| RF-Generator | 40 MHz |
| Replicates for each analysis run | 3 |
| Sample uptake delay | 30 s |
| Viewing mode | Axial |
| Spray chamber type | Cyclonic |
| Sample propulsion | Peristaltic pump, three channel |
| Torch type | Fassel type |
| Detector | Segmented-array charge-coupled (SCD) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zheng, S.; Yang, Q.; Chen, S.; Huang, L. Distinguishing Astragalus mongholicus and Its Planting Soil Samples from Different Regions by ICP-AES. Molecules 2016, 21, 482. https://doi.org/10.3390/molecules21040482
Li L, Zheng S, Yang Q, Chen S, Huang L. Distinguishing Astragalus mongholicus and Its Planting Soil Samples from Different Regions by ICP-AES. Molecules. 2016; 21(4):482. https://doi.org/10.3390/molecules21040482
Chicago/Turabian StyleLi, Lin, Sihao Zheng, Qingzhen Yang, Shilin Chen, and Linfang Huang. 2016. "Distinguishing Astragalus mongholicus and Its Planting Soil Samples from Different Regions by ICP-AES" Molecules 21, no. 4: 482. https://doi.org/10.3390/molecules21040482
APA StyleLi, L., Zheng, S., Yang, Q., Chen, S., & Huang, L. (2016). Distinguishing Astragalus mongholicus and Its Planting Soil Samples from Different Regions by ICP-AES. Molecules, 21(4), 482. https://doi.org/10.3390/molecules21040482