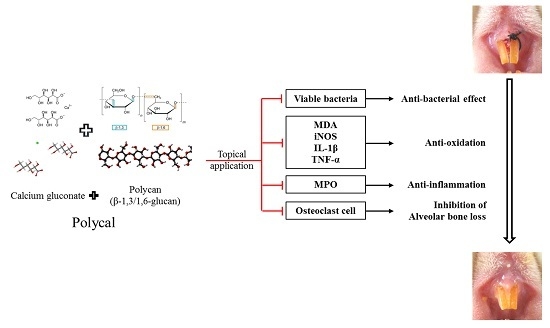
The Effects of Topical Application of Polycal (a 2:98 (g/g) Mixture of Polycan and Calcium Gluconate) on Experimental Periodontitis and Alveolar Bone Loss in Rats
Abstract
:1. Introduction
2. Results
2.1. Changes in Bodyweight
2.2. Alveolar Bone Loss Scores
2.3. Gingival Viable Bacteria Counts
2.4. Anti-Inflammatory and Anti-Oxidant Effects
2.4.1. Changes on the Gingival MPO Activities
2.4.2. Changes on the Gingival IL-1β Levels
2.4.3. Changes on the Gingival TNF-α Levels
2.4.4. Changes on the Gingival MDA Levels
2.4.5. Changes on the Gingival iNOS Activities
2.5. Histopathology of the Maxillary Regions
2.5.1. Changes on the Numbers of Inflammatory Cells Infiltrated in Gingival Tissues
2.5.2. Changes on the Collagen Fiber Occupied Regions in Gingival Tissues
2.5.3. Changes on the Alveolar Bone Volumes
2.5.4. Changes on the Osteoclast Cells
2.6. Histological Scores
3. Discussion
4. Materials and Methods
4.1. Animals and Husbandry
4.2. Preparations and Administration of Test Materials
4.3. Induction of EPD
4.4. Measurement of Bodyweight and Alveolar Bone Loss
4.5. Microbiological Analysis
4.6. Measurement of Myeloperoxidase Activity
4.7. Detection of IL-1β and TNF-α in Rat maxillary Gingival Tissue
4.8. MDA Measurement
4.9. iNOS Activity Measurement
4.10. Histopathology
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BHI | Brain heart infusion broth |
| EPD | Experimental periodontitis |
| GLP | Good Laboratory Practice |
| HE | Hematoxylin-eosin |
| HTAB | Hexadecyltrimethyl-ammonium bromide |
| IL | Interleukin |
| IND | Indomethacin, reference |
| iNOS | Inducible nitric oxide synthase |
| MDA | Malondialdehyde |
| MPO | Myeloperoxidase |
| NSAIDs | Non-steroid anti-inflammatory drugs |
| OS/BS | Percentages of osteoclast cells occupied regions on the alveolar bone surface |
| PMNs | Polymorphneutrophils |
| Polycal | Polycan and calcium gluconate 2:98 (g/g) mixture, test materials |
| SD | Standard deviation |
| SPF | Specific pathogen free |
| TNF | Tumor necrosis factor |
| VAF | Virus antibody free |
References
- Chambrone, L.A.; Chambrone, L. Tooth loss in well-maintained patients with chronic periodontitis during long-term supportive therapy in Brazil. J. Clin. Periodontol 2006, 33, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Sallay, K.; Sanavi, F.; Ring, I.; Pham, P.; Behling, U.H.; Nowotny, A. Alveolar bone destruction in the immunosuppressed rat. J. Periodontal Res. 1982, 17, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Samejima, Y.; Ebisu, S.; Okada, H. Effect of infection with Eikenella corrodens on the progression of ligature-induced periodontitis in rats. J. Periodontal Res. 1990, 25, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.A.; Rao, V.S.; Carvalho, C.B.; Bezerra-Filho, J.G.; Fonseca, S.G.; Vale, M.L.; Montenegro, D.; Cunha, F.; Ribeiro, R.A.; Brito, G.A. Lippia sidoides and Myracrodruon urundeuva gel prevents alveolar bone resorption in experimental periodontitis in rats. J. Ethnopharmacol. 2007, 113, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.M.; Rocha, F.A.; Chaves, H.V.; Carvalho, C.B.; Ribeiro, R.A.; Brito, G.A. Effect of sodium alendronate on alveolar bone resorption in experimental periodontitis in rats. J. Periodontol 2005, 76, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Lohinai, Z.; Benedek, P.; Fehér, E.; Györfi, A.; Rosivall, L.; Fazekas, A.; Salzman, A.L.; Szabó, C. Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. Br. J. Pharm. 1998, 123, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Cho, H.R.; Sung, Y.S.; Kang, S.J.; Lee, Y.J. Effects of calcium gluconate on experimental periodontitis and alveolar bone loss in rats. Basic Clin. Pharmacol. Toxicol 2011, 108, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kang, S.J.; Kim, J.W.; Cho, H.R.; Moon, S.B.; Kim, K.Y.; Lee, H.S.; Han, C.H.; Ku, S.K.; Lee, Y.J. Effects of Polycan, α β-glucan, on experimental periodontitis and alveolar bone loss in Sprague-Dawley rats. J. Periodontal Res. 2012, 47, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.P.; Kim, J.M.; Shin, H.D.; Kim, T.K.; Chang, H.J.; Park, B.R.; Lee, J.W. Production of-1, 3/1, 6-glucan by Aureobasidium pullulans SM-2001. Korean J. Bitechnol. Bioeng. 2002, 17, 376–380. [Google Scholar]
- Song, H.B.; Park, D.C.; Gyung, M.D.; Hwang, S.L.; Lee, W.K.; Kang, H.S.; Park, B.R.; Jang, H.J.; Son, C.W.; Park, E.K. Effect of exopolymers of Aureobasidium pullulans on improving osteoporosis induced in ovariectomized mice. J. Microbiol. Biotechnol. 2006, 16, 37–45. [Google Scholar]
- Shin, H.D.; Yang, K.J.; Park, B.R.; Son, C.W.; Jang, H.J.; Ku, S.K. Antiosteoporotic effect of Polycan, b-glucan from Aureobasidium, in ovariectomized osteoporotic mice. Nutrition 2007, 23, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Cho, H.R.; Moon, S.B.; Shin, H.D.; Yang, K.J.; Park, B.R.; Jang, H.J.; Kim, L.S.; Lee, H.S.; Ku, S.K. Effects of β-glucan from Aureobasidium pullulans on acute inflammation in mice. Arch. Pharm. Res. 2007, 30, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Cho, H.R.; Moon, S.B.; Shin, H.D.; Yang, K.J.; Park, B.R.; Jang, H.-J.; Kim, L.S.; Lee, H.S.; Ku, S.K. Effect of exopolymers from Aureobasidium pullulans on formalin-induced chronic paw inflammation in mice. J. Microbiol. Biotechnol. 2006, 16, 1954–1960. [Google Scholar]
- Ku, S.K.; Lee, Y.J.; Lee, S.D.; Cho, H.R.; Moon, S.B.; Kim, K.Y.; Kwon, Y.S.; Kim, J.W. Nephroprotective effect of POLYCAN on acute renal failure induced by cisplatin in rats. ISRN. Vet. Sci. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Hendry, J.A.; Jeansonne, B.G.; Dummett, C.O., Jr.; Burrell, W. Comparison of calcium hydroxide and zinc oxide and eugenol pulpectomies in primary teeth of dogs. Oral. Surg. Oral. Med. Oral. Pathol. 1982, 54, 445–451. [Google Scholar] [CrossRef]
- Piller, N.B. Assessment of the anti-inflammatory action of calcium dobesilate. Effect on macrophages attaching to subcutaneously implanted coverslips in guinea pigs. Arzneimittelforschung 1990, 40, 698–700. [Google Scholar] [PubMed]
- Smith, M.M.; Ghosh, P.; Numata, Y.; Bansal, M.K. The effects of orally administered calcium pentosan polysulfate on inflammation and cartilage degradation produced in rabbit joints by intraarticular injection of a hyaluronate-polylysine complex. Arthritis Rheum. 1994, 37, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Karnad, A.; Patil, P.; Majagi, S. Calcium enhances antiinflammatory activity of aspirin in albino rats. Indian J. Pharmacol. 2006, 38, 397–402. [Google Scholar]
- Sohn, K.C.; Kang, S.J.; Kim, J.W.; Kim, K.Y.; Ku, S.K.; Lee, Y.J. Effects of Calcium Gluconate, a Water Soluble Calcium Salt on the Collagen-Induced DBA/1J Mice Rheumatoid Arthritis. Biomol. Ther. 2013, 21, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, J.W.; Kim, K.Y.; Cho, H.R.; Choi, I.S.; Ku, S.K. Antiosteoporotic effects of Polycan in combination with calcium lactate-gluconate in ovariectomized rats. Exp. Ther. Med. 2014, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Choi, J.S.; Ha, Y.M.; Choi, I.S.; Kim, K.Y.; Cho, H.R.; Rha, C.H.; Ku, S.K. Single oral dose toxicity test of polycalcium, a mixed composition of polycan and calcium lactate-gluconate 1:9 (G/G) in SD rat. Pak. J. Pharm. Sci. 2013, 26, 1141–1150. [Google Scholar] [PubMed]
- Lee, W.H.; Kim, K.H.; Kang, S.J.; Lee, Y.J.; Ku, S.K. Synergic Effects of Mixed Formula Consisted of Polycan and Calcium-gluconate on the Experimental Periodontitis and Alveolar Bone Loss in Rats. J. Soc. Prev. Korean Med. 2014, 18, 125–138. [Google Scholar]
- Azoubel, M.C.; Menezes, A.M.; Bezerra, D.; Oriá, R.B.; Ribeiro, R.A.; Brito, G.A. Comparison of etoricoxib and indomethacin for the treatment of experimental periodontitis in rats. Braz. J. Med. Biol. Res. 2007, 40, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ximenez-Fyvie, L.A.; Almaguer-Flores, A.; Jacobo-Soto, V.; Lara-Cordoba, M.; Moreno-Borjas, J.Y.; Alcantara-Maruri, E. Subgingival microbiota of periodontally untreated Mexican subjects with generalized aggressive periodontitis. J. Clin. Periodontol 2006, 33, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Sandven, P. β-1,3-Glucan reduces growth of Mycobacterium tuberculosis in macrophage cultures. FEMS Immunol. Med. Microbiol. 2002, 33, 41–45. [Google Scholar] [CrossRef]
- Dobšíková, R.; Blahová, J.; Mikuliková, I.; Modrá, H.; Prášková, E.; Svobodová, Z.; Skorič, M.; Jarkovský, J.; Siwicki, A.K. The effect of oyster mushroom b-1.3/1.6-D-glucan and oxytetracycline antibiotic on biometrical, haematological, biochemical, and immunological indices, and histopathological changes in common carp (Cyprinus carpio L.). Fish. Shellfish. Immunol. 2013, 35, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.K.; Mandal, A.K.; Chakraborti, S.; Dey, B.; Chakraborty, R.; Islam, S.S. Green synthesis of silver nanoparticles using glucan from mushroom and study of antibacterial activity. Int. J. Biol. Macromol. 2013, 62, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pope, R.M. Phagocytes: mechanisms of inflammation and tissue destruction. Rheum. Dis. Clin. N. Am. 2004, 30, 19–39. [Google Scholar] [CrossRef]
- Zimmerman, B.J.; Grisham, M.B.; Granger, D.N. Role of oxidants in ischemia/reperfusion-induced granulocyte infiltration. Am. J. Physiol. 1990, 258, 185–190. [Google Scholar]
- Işeri, S.O.; Sener, G.; Yüksel, M.; Contuk, G.; Cetinel, S.; Gedik, N.; Yegen, B.C. Ghrelin against alendronate-induced gastric damage in rats. J. Endocrinol. 2005, 187, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Samira, S.; Ferrand, C.; Peled, A.; Nagler, A.; Tovbin, Y.; Ben-Hur, H.; Taylor, N.; Globerson, A.; Lapidot, T. Tumor necrosis factor promotes human T-cell development in nonobese diabetic/severe combined immunodeficient mice. Stem. Cells 2004, 22, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A. Lymphokines and cytokines. In Immunology, an Introduction, 4th ed.; Tizard, I.R., Ed.; Saunders College Pub.: Philadelphia, PA, USA, 1995; pp. 155–169. [Google Scholar]
- Unanue, E.R. The Mononuclear-Phagocytic sytem. In Immunology, an Introduction, 4th ed.; Tizard, I.R., Ed.; Saunders College Pub.: Philadelphia, PA, USA, 1995; pp. 61–74. [Google Scholar]
- Assuma, R.; Oates, T.; Cochran, D.; Amar, S.; Graves, D.T. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 1998, 160, 403–409. [Google Scholar] [PubMed]
- Di Paola, R.; Mazzon, E.; Zito, D.; Maiere, D.; Britti, D.; Genovese, T.; Cuzzocrea, S. Effects of Tempol, a membrane-permeable radical scavenger, in a rodent model periodontitis. J. Clin. Periodontol 2005, 32, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, C.; Du, G.; Cao, Z. Protective effects of baicalin on ligature-induced periodontitis in rats. J. Periodontal Res. 2008, 43, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Toker, H.; Ozdemir, H.; Eren, K.; Ozer, H.; Sahin, G. N-Acetylcysteine, a thiol antioxidant, decreases alveolar bone loss in experimental periodontitis in rats. J. Periodontol. 2009, 80, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Zingarelli, B.; Hake, P.; Salzman, A.L.; Szabó, C. Antiinflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Radic. Biol. Med. 1998, 24, 450–459. [Google Scholar] [CrossRef]
- Di Paola, R.; Marzocco, S.; Mazzon, E.; Dattola, F.; Rotondo, F.; Britti, D.; De Majo, M.; Genovese, T.; Cuzzocrea, S. Effect of aminoguanidine in ligature-induced periodontitis in rats. J. Dent. Res. 2004, 83, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C. Alterations in nitric oxide production in various forms of circulatory shock. New Horiz. 1995, 3, 2–32. [Google Scholar] [PubMed]
- Southan, G.J.; Szabó, C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem. Pharmacol. 1996, 51, 383–394. [Google Scholar] [CrossRef]
- Bezerra, J.P.; da Silva, L.R.; de Alvarenga Lemos, V.A.; Duarte, P.M.; Bastos, M.F. Administration of high doses of caffeine increases alveolar bone loss in ligature-induced periodontitis in rats. J. Periodontol. 2008, 79, 2356–2360. [Google Scholar] [CrossRef] [PubMed]
- Holanda Pinto, S.A.; Pinto, L.M.; Cunha, G.M.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Anti-inflammatory effect of a, b-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology 2008, 16, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Leitão, R.F.; Ribeiro, R.A.; Chaves, H.V.; Rocha, F.A.; Lima, V.; Brito, G.A. Nitric oxide synthase inhibition prevents alveolar bone resorption in experimental periodontitis in rats. J. Periodontol. 2005, 76, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.M.; Taubman, M.A.; Smith, D.J. The natural history of periodontal bone loss in germfree and gnotobiotic rats infected with periodontopathic microorganisms. J. Periodontal Res. 1978, 13, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.













| Items | Body Weights (g) | Body Weight Gains [B − A] | ||||
|---|---|---|---|---|---|---|
| Groups | Before Ligation | At Ligation | At Initiation of Test Article Treatment [A] | At Last Administration of Test Article [B] | ||
| Controls | ||||||
| Intact | 250.13 ± 11.99 | 221.00 ± 14.20 | 248.50 ± 13.10 | 279.63 ± 11.83 | 31.13 ± 4.16 | |
| EPD | 250.63 ± 11.76 | 222.75 ± 12.94 | 241.13 ± 10.83 | 247.00 ± 10.81 a | 5.88 ± 4.70 a | |
| Reference | ||||||
| IND | 249.75 ± 14.03 | 221.25 ± 14.91 | 242.00 ± 16.37 | 225.63 ± 14.99 a,c | 16.38 ± 5.10 a,c | |
| Polycal concentrations | ||||||
| H: 50 mg/mL | 250.38 ± 17.52 | 220.88 ± 17.72 | 242.00 ± 15.69 | 265.75 ± 16.82 b,c | 23.75 ± 4.80 a,c | |
| M: 25 mg/mL | 250.38 ± 14.38 | 222.63 ± 16.48 | 241.75 ± 14.10 | 262.00 ± 8.78 a,d | 20.25 ± 6.30 a,c | |
| L: 12.5 mg/mL | 250.25 ± 14.92 | 222.13 ± 16.03 | 242.88 ± 13.55 | 259.50 ± 9.55 a | 16.63 ± 6.57 a,c | |
| Items | Gingival Areas | Alveolar Bone Areas | ||||
|---|---|---|---|---|---|---|
| Groups | Inflammatory cells (Cells/mm2) | Collagen Percentages (%/mm2) | Alveolar Bone volume (%) | Osteoclast Cell (cells/mm) | OS/BS (%) | |
| Controls | ||||||
| Intact | 9.38 ± 3.74 | 68.06 ± 11.52 | 65.68 ± 10.19 | 5.88 ± 1.73 | 1.74 ± 0.91 | |
| EPD | 1110.00 ± 265.89 a | 21.66 ± 10.06 a | 33.17 ± 10.29 a | 38.38 ± 10.39 a | 35.21 ±10.36 a | |
| Reference | ||||||
| IND | 226.38 ± 73.92 a,b | 49.65 ± 11.52 a,b | 49.70 ± 10.85 a,b | 25.38 ± 5.01 a,b | 20.05 ± 4.18 a,b | |
| Polycal concentrations | ||||||
| H: 50 mg/mL | 137.00 ± 45.42 a,b | 61.28 ± 12.05 b | 56.66 ± 11.61 b | 15.38 ± 5.18 a,b | 11.69 ± 3.65 a,b | |
| M: 25 mg/mL | 234.25 ± 76.65 a,b | 50.13 ± 12.77 a,b | 50.01 ± 11.16 a,b | 23.88 ± 4.97 a,b | 18.95 ± 4.89 a,b | |
| L: 12.5 mg/mL | 488.75 ± 109.65 a,b | 42.33 ± 15.22 a,b | 47.44 ± 7.59 a,b | 26.88 ± 5.99 a,b | 22.66 ± 4.59 a,b | |
| Scores | Remarks |
|---|---|
| 0 | Absence or only a discrete cellular infiltration (inflammatory cell infiltration is sparse and restricted to the region of the marginal gingival), preserved alveolar process and cementum |
| 1 | Moderate cellular infiltration (inflammatory cellular infiltration present all over the insert gingival), some but minor alveolar process resorption and Intact cementum |
| 2 | Accentuated cellular infiltration (inflammatory cellular infiltration present in both gingival and periodontal ligament), accentuated degradation of the alveolar process and partial destruction of cementum |
| 3 | Accentuated cellular infiltrate, complete resorption of the alveolar process and severe destruction of cementum |
| Max = 3 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-I.; Kang, S.-J.; Han, C.-H.; Kim, J.-W.; Song, C.-H.; Lee, S.-N.; Ku, S.-K.; Lee, Y.-J. The Effects of Topical Application of Polycal (a 2:98 (g/g) Mixture of Polycan and Calcium Gluconate) on Experimental Periodontitis and Alveolar Bone Loss in Rats. Molecules 2016, 21, 527. https://doi.org/10.3390/molecules21040527
Park S-I, Kang S-J, Han C-H, Kim J-W, Song C-H, Lee S-N, Ku S-K, Lee Y-J. The Effects of Topical Application of Polycal (a 2:98 (g/g) Mixture of Polycan and Calcium Gluconate) on Experimental Periodontitis and Alveolar Bone Loss in Rats. Molecules. 2016; 21(4):527. https://doi.org/10.3390/molecules21040527
Chicago/Turabian StylePark, Sang-In, Su-Jin Kang, Chang-Hyun Han, Joo-Wan Kim, Chang-Hyun Song, Sang-Nam Lee, Sae-Kwang Ku, and Young-Joon Lee. 2016. "The Effects of Topical Application of Polycal (a 2:98 (g/g) Mixture of Polycan and Calcium Gluconate) on Experimental Periodontitis and Alveolar Bone Loss in Rats" Molecules 21, no. 4: 527. https://doi.org/10.3390/molecules21040527
APA StylePark, S. -I., Kang, S. -J., Han, C. -H., Kim, J. -W., Song, C. -H., Lee, S. -N., Ku, S. -K., & Lee, Y. -J. (2016). The Effects of Topical Application of Polycal (a 2:98 (g/g) Mixture of Polycan and Calcium Gluconate) on Experimental Periodontitis and Alveolar Bone Loss in Rats. Molecules, 21(4), 527. https://doi.org/10.3390/molecules21040527