Design, Synthesis and Evaluation of Antiproliferative Activity of New Benzimidazolehydrazones
Abstract
:1. Introduction
2. Results
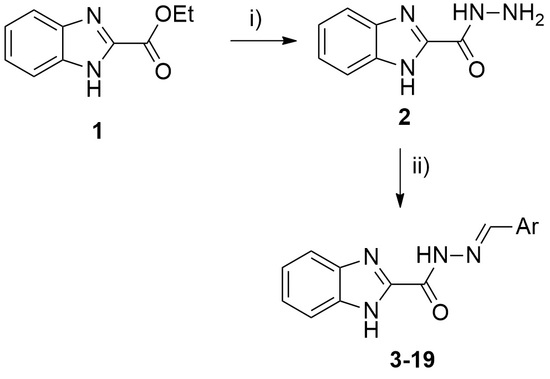
2.1. Chemistry
2.2. Antiproliferative Activity
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Synthesis
4.2.2. General Procedure for the Synthesis of Hydrazones 3–19
4.3. Antiproliferative Activity
4.3.1. Cell Lines
4.3.2. Cell Proliferation
4.3.3. IC50 Determination
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADME | Adsorption, distribution, metabolism and elimination |
| IR | infrared spectra |
| NMR | Nuclear magnetic resonance |
| MS | Mass spectra |
| TPSA | Total polar surface area |
References
- Yadav, G.; Ganguly, S. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem. 2015, 97, 419–443. [Google Scholar] [PubMed]
- Gowda, N.R.; Kavitha, C.V.; Chiruvella, K.K.; Joy, O.; Rangappa, K.S.; Raghavan, S.C. Synthesis and biological evaluation of novel 1-(4-methoxyphenethyl)-1H-benzimidazole-5-carboxylic acid derivatives and their precursors as antileukemic agents. Bioorg. Med. Chem. Lett. 2009, 19, 4594–4600. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, C.; Gao, C.; Zhang, C.; Luan, X.; Chen, X.; Liu, H.; Chen, Y.; Jiang, Y. Discovery of benzimidazole derivatives as novel multi-target EGFR, VEGFR-2 and PDGFR kinase inhibitors. Bioorg. Med. Chem. 2011, 19, 4529–4535. [Google Scholar] [CrossRef] [PubMed]
- Determann, R.; Dreher, J.; Baumann, K.; Preu, L.; Jones, P.G.; Totzke, F.; Schächtele, C.; Kubbutat, M.H.; Kunick, C. 2-Anilino-4-(benzimidazol-2-yl)pyrimidines—A multikinase inhibitor scaffold with antiproliferative activity toward cancer cell lines. Eur. J. Med. Chem. 2012, 53, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, B.; Zhang, B.; Sun, Q.; Li, L.; Li, X.; Chen, C.; Tan, C.; Liu, H.; Jiang, Y. Synthesis and biological evaluation of benzimidazole acridine derivatives as potential DNA-binding and apoptosis-inducing agents. Bioorg. Med. Chem. 2015, 23, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Han, C.; Zuo, D.; Zhai, M.; Li, Z.; Zhang, Q.; Zhai, Y.; Jiang, X.; Bao, K.; Wu, Y.; et al. Synthesis and evaluation of benzimidazole carbamates bearing indole moieties for antiproliferative and antitubulin activities. Eur. J. Med. Chem. 2014, 87, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Maji, B.; Kumar, K.; Kaulage, M.; Muniyappa, K.; Bhattacharya, S. Design and Synthesis of New Benzimidazole–Carbazole Conjugates for the Stabilization of Human Telomeric DNA, Telomerase Inhibition, and Their Selective Action on Cancer Cells. J. Med. Chem. 2014, 57, 6973–6988. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Tandon, V. Synthesis and biological activity of novel inhibitors of topoisomerase I: 2-Aryl-substituted 2-bis-1H-benzimidazoles. Eur. J. Med. Chem. 2011, 46, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.K.; Ali, M.A.; Wei, A.C.; Shirazi, A.N.; Parang, K.; Choon, T.S. Benzimidazoles as new scaffold of sirtuin inhibitors: Green synthesis, in vitro studies, molecular docking analysis and evaluation of their anti-cancer properties. Eur. J. Med. Chem. 2014, 83, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.K.; Ali, M.A.; Wei, A.C.; Choon, T.S.; Osman, H.; Parang, K.; Shirazi, A.N. Synthesis and evaluation of novel benzimidazole derivatives as sirtuin inhibitors with antitumor activities. Bioorg. Med. Chem. 2014, 22, 703–710. [Google Scholar]
- Salum, L.B.; Mascarello, A.; Canevarolo, R.R.; Altei, W.F.; Laranjeira, A.B.; Neuenfeldt, P.D.; Stumpf, T.R.; Chiaradia-Delatorre, L.D.; Vollmer, L.L.; Daghestani, H.N.; et al. N-(1′-naphthyl)-3,4,5-trimethoxybenzohydrazide as microtubule destabilizer: Synthesis, cytotoxicity, inhibition of cell migration and in vivo activity against acute lymphoblastic leukemia. Eur. J. Med. Chem. 2015, 96, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Kaplánek, R.; Jakubek, M.; Rak, J.; Kejík, Z.; Havlík, M.; Dolenský, B.; Frydrych, I.; Hajdúch, M.; Kolář, M.; Bogdanová, K.; et al. Caffeine–hydrazones as anticancer agents with pronounced selectivity toward T-lymphoblastic leukaemia cells. Bioorg. Chem. 2015, 60, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Xu, C.; Ji, X.; Hu, G.; Ren, L.; Liu, Y.; Li, R.; Gong, P.; Sun, T. Design and optimization of novel 4-(2-fluorophenoxy)quinoline derivatives bearing a hydrazone moiety as c-Met kinase inhibitors. Eur. J. Med. Chem. 2014, 87, 508–518. [Google Scholar]
- Potůčková, E.; Hrušková, K.; Bureš, J.; Kovaříková, P.; Špirková, I.A.; Pravdíková, K.; Kolbabová, L.; Hergeselová, T.; Hašková, P.; Jansová, H.; et al. Structure-activity relationships of novel salicylaldehyde isonicotinoyl hydrazone (SIH) analogs: Iron chelation, anti-oxidant and cytotoxic properties. PLoS ONE 2014, 9, e112059. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Fan, C.-D.; Zhao, B.-X.; Shin, D.-S.; Miao, J.-Y. Synthesis and structure–activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide hydrazone derivatives as potential agents against A549 lung cancer cells. Eur. J. Med. Chem. 2008, 43, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Onnis, V.; Cocco, M.T.; Fadda, R.; Congiu, C. Synthesis and evaluation of anticancer activity of 2-arylamino-6-trifluoromethyl-3-(hydrazonocarbonyl)pyridines. Bioorg. Med. Chem. 2009, 17, 6158–6165. [Google Scholar] [CrossRef] [PubMed]
- Congiu, C.; Onnis, V. Synthesis and biological evaluation of novel acylhydrazone derivatives as potential antitumor agents. Bioorg. Med. Chem. 2013, 21, 6592–6599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Basavarajaaswamy, G.; Rai, M.V.; Poojary, B.; Pai, V.R.; Shruthi, N.; Bhat, M. Rapid ‘one-pot’ synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. Lett. 2015, 25, 1420–1426. [Google Scholar]
- Liu, T.; Sun, C.; Xing, X.; Jing, L.; Tan, R.; Luo, Y.; Zhao, Y. Synthesis and evaluation of 2-[2-(phenylthiomethyl)-1H-benzo[d]imidazol-1-yl)acetohydrazide derivatives as antitumor agents. Bioorg. Med. Chem. Lett. 2012, 22, 3122–3125. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, S.; Vertuani, S.; Scalambra, E. Dualistic Molecules Having UV Radiation Filtrating Ability at Wide Spectrum and Potent Damping Activity of the Reactivity of Free Radicals (Radicals Scavenging). International Patent Application WO/2013/102843, 11 July 2013. [Google Scholar]
- Lima, P.C.; Lima, L.M.; da Silva, K.C.M.; Leda, P.H.O.; de Miranda, A.L.P.; Fraga, C.A.M.; Eliezer, J.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kapple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Graubaum, H.; Martin, D. Reactions of benzimidazole-2-carbohydrazide with electrophilic compounds. J. Prakt. Chem. 1986, 328, 515–521. [Google Scholar] [CrossRef]
- Harisha, R.S.; Hosamani, K.M.; Keri, R.S. Synthesis, in vitro microbial and cytotoxic studies of new benzimidazole derivatives. Arch. Pharm. 2009, 342, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 3–19 are available from the authors.

| Compd. | Ar | IC50 (μM) a | |||
|---|---|---|---|---|---|
| L1210 | CEM | HeLa | Mia-Paca-2 | ||
| 3 | 2-OH-phenyl | 5.7 ± 0.9 | 4.4 ± 0.2 | 12 ± 0.7 | 34 ± 0.9 |
| 4 | 3-OH-phenyl | 94 ± 7 | 98 ± 10 | 88 ± 12.5 | >100 |
| 5 | 4-OH-phenyl | 47 ± 2 | 85 ± 0.01 | >100 | >100 |
| 6 | 2,4-(OH)2-phenyl | 2.6 ± 0.9 | 2.6 ± 1.0 | 4.7 ± 1.6 | 21 ± 12 |
| 7 | 2,5-(OH)2-phenyl | 22 ± 7 | 4.9 ± 1.2 | 77 ± 18 | >100 |
| 8 | 2,3,4-(OH)3-phenyl | 47 ± 7 | 20 ± 8 | 97 ± 28 | >100 |
| 9 | 2,4,6-(OH)3-phenyl | 90 ± 7 | >100 | >100 | >100 |
| 10 | 2-OH-4-OMe-phenyl | 1.6 ± 0.9 | 0.98 ± 0.02 | 4.0 ± 0.4 | 6.3 ± 3.2 |
| 11 | 2-OH-3-OEt-phenyl | 5.9 ± 2.4 | 6.3 ± 1.9 | 22 ± 0.02 | 23 ± 8 |
| 12 | 3-OH-4-OMe-phenyl | 92 ± 4 | 57 ± 3.7 | >100 | >100 |
| 13 | 2-OH-4-N(Et)2-phenyl | 14 ± 2 | 4.8 ± 0.8 | 23 ± 3 | 40 ± 6 |
| 14 | 2-OH-5-Cl-phenyl | 7.4 ± 2.5 | 1.8 ± 0.6 | 4.8 ± 0.9 | 9.2 ± 4.0 |
| 15 | 2-OH-5-Br-phenyl | 5.0 ± 2.8 | 1.8 ± 0.9 | 4.9 ± 0.4 | 35 ± 5 |
| 16 | 2-OH-naphtyl | 2.9 ± 1.3 | 1.0 ± 0.01 | 2.5 ± 1.4 | 7.9 ± 0.3 |
| 17 | phenyl | >250 | >250 | >250 | 220 ± 37 |
| 18 | 4-OMe-phenyl | >250 | >250 | >250 | >250 |
| 19 | napht-1-yl | 240±13 | >250 | >250 | >250 |
| Compd. | TPSA | n-ROTB | MV | MW | miLogP | n-ON | n-OHNH | n-Viol |
|---|---|---|---|---|---|---|---|---|
| 3 | 90.37 | 3 | 242.94 | 280.29 | 2.64 | 6 | 3 | 0 |
| 6 | 110.60 | 3 | 250.96 | 296.29 | 2.13 | 7 | 4 | 0 |
| 7 | 110.60 | 3 | 250.96 | 296.29 | 2.13 | 7 | 4 | 0 |
| 8 | 130.83 | 3 | 258.97 | 312.29 | 1.67 | 8 | 5 | 0 |
| 10 | 99.61 | 4 | 268.48 | 310.31 | 2.677 | 7 | 3 | 0 |
| 11 | 99.61 | 5 | 285.29 | 324.34 | 2.62 | 7 | 3 | 0 |
| 13 | 93.61 | 6 | 322.45 | 351.41 | 3.47 | 7 | 3 | 0 |
| 14 | 90.37 | 3 | 256.47 | 314.73 | 3.29 | 6 | 3 | 0 |
| 15 | 90.37 | 3 | 260.82 | 359.18 | 3.42 | 6 | 3 | 0 |
| 16 | 90.37 | 3 | 286.93 | 330.35 | 3.79 | 6 | 3 | 0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onnis, V.; Demurtas, M.; Deplano, A.; Balboni, G.; Baldisserotto, A.; Manfredini, S.; Pacifico, S.; Liekens, S.; Balzarini, J. Design, Synthesis and Evaluation of Antiproliferative Activity of New Benzimidazolehydrazones. Molecules 2016, 21, 579. https://doi.org/10.3390/molecules21050579
Onnis V, Demurtas M, Deplano A, Balboni G, Baldisserotto A, Manfredini S, Pacifico S, Liekens S, Balzarini J. Design, Synthesis and Evaluation of Antiproliferative Activity of New Benzimidazolehydrazones. Molecules. 2016; 21(5):579. https://doi.org/10.3390/molecules21050579
Chicago/Turabian StyleOnnis, Valentina, Monica Demurtas, Alessandro Deplano, Gianfranco Balboni, Anna Baldisserotto, Stefano Manfredini, Salvatore Pacifico, Sandra Liekens, and Jan Balzarini. 2016. "Design, Synthesis and Evaluation of Antiproliferative Activity of New Benzimidazolehydrazones" Molecules 21, no. 5: 579. https://doi.org/10.3390/molecules21050579
APA StyleOnnis, V., Demurtas, M., Deplano, A., Balboni, G., Baldisserotto, A., Manfredini, S., Pacifico, S., Liekens, S., & Balzarini, J. (2016). Design, Synthesis and Evaluation of Antiproliferative Activity of New Benzimidazolehydrazones. Molecules, 21(5), 579. https://doi.org/10.3390/molecules21050579