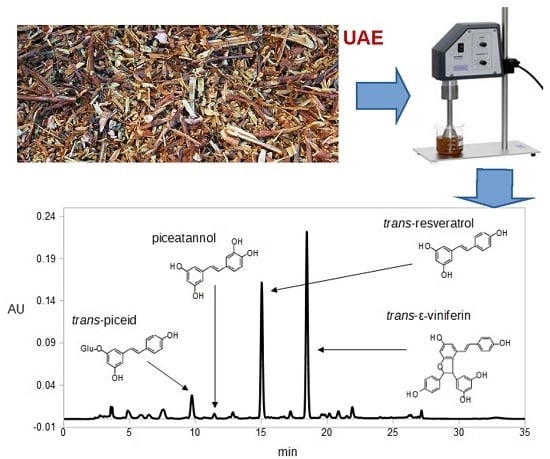
Ultrasound-Assisted Extraction of Stilbenes from Grape Canes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Study of Sample Pre-Treatment and Storage Conditions
2.2. Stability of Stilbenes
2.3. Solvent Selection
2.4. Sample-Solvent Ratio
2.5. Extraction Time
2.6. Analytical Characteristics of the Method
2.7. Comparison of the Method with Reference Solid-Liquid Extraction
2.8. Determination of Stilbenes in Grape Cane Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Extraction Process
3.3.1. Ultrasound-Assisted Extraction (UAE)
3.3.2. Reference Solid-Liquid Extraction (SLE)
3.4. Liquid Chromatography System
3.5. Statistical Software
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shen, T.; Wang, X.-N.; Lou, H.-X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Villaverde, V.; Voinesco, F.; Viret, O.; Spring, J.-L.; Gindro, K. The effectiveness of stilbenes in resistant Vitaceae: Ultrastructural and biochemical events during Plasmopara viticola infection process. Plant Physiol. Biochem. 2011, 49, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Asensi, M.; Medina, I.; Ortega, A.; Carretero, J.; Bano, M.C.; Obrador, E.; Estrela, J.M. Inhibitory effects of trans-resveratrol analogs molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Mol. Nutr. Food Res. 2008, 52, 538–548. [Google Scholar]
- Hao, H.D.; He, L.R. Mechanisms of cardiovascular protection by resveratrol. J. Med. Food 2004, 7, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- State of the Vitivinicultre World Market April 2016. International Organization of World Vitiviniculture (OIV). Available online: http://www.oiv.int/public/medias/4587/oiv-noteconjmars2016-en.pdf (accessed on 25 April 2016).
- Karacabey, E.; Mazza, G. Optimization of solid-liquid extraction of resveratrol and other phenolic compounds from milled grape canes (Vitis vinifera). J. Agric. Food Chem. 2008, 56, 6318–6325. [Google Scholar] [CrossRef] [PubMed]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Baydar, N.G. Chemical composition of grape canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- Careri, M.; Corradini, C.; Elviri, L.; Nicoletti, E.; Zagnoni, E.I. Direct HPLC analysis of quercetin and trans-resveratrol in red wine, grape, and winemaking byproducts. J. Agric. Food Chem. 2003, 51, 5226–5231. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Bioactive non-coloured polyphenols content of grapes, wines and vinification by-products: Evaluation of the antioxidant activities of their extracts. Food Res. Int. 2010, 43, 805–813. [Google Scholar] [CrossRef]
- Ji, M.; Li, Q.; Ji, H.; Lou, H. Investigation of the distribution and season regularity of resveratrol in Vitis amurensis via HPLC-DAD-MS/MS. Food Chem. 2014, 142, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Baderschneider, B.; Winterhalter, P. Isolation and characterization of novel stilbene derivatives from Riesling wine. J. Agric. Food Chem. 2000, 48, 2681–2686. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Yanbin, L.; Liyan, J.; Bin, W.; Feiying, Z.; Yuanjiang, P. Preparative isolation and purification of antioxidative stilbene oligomers from Vitis chunganeniss using high-speed counter-current chromatography in stepwise elution mode. J. Sep. Sci. 2009, 32, 2339–2345. [Google Scholar]
- Cho, J.-Y.; Chun, H.S.; Lee, S.K.; Min, H.-Y. Ultrasonication-assisted extraction of resveratrol from grapes. J. Food Eng. 2006, 77, 725–730. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; de la Ossa, E.J.M.; Roldán, A.; de Ory, I.; Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Delgado-Torre, M.P.; Ferreiro-Vera, C.; Priego-Capote, F.; Pérez-Juan, P.M.; de Castro, M.D.L. Comparison of accelerated methods for the extraction of phenolic compounds from different vine-shoot cultivars. J. Agric. Food Chem. 2012, 60, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Lambert, C.; Richard, T.; Renouf, E.; Bisson, J.; Waffo-Téguo, P.; Bordenave, L.; Ollat, N.; Mérillon, J.-M.; Cluzet, S. Comparative analyses of stilbenoids in canes of major Vitis vinifera L. cultivars. J. Agric. Food Chem. 2013, 61, 11392–11399. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; von Baer, C.; Mardones, A.; Wilkens, K.; Wernekinck, A.; Damm, S.; Macke, T.; Gorena, P.; Winterhalter, P. Stilbene levels in grape cane of different cultivars in Southern Chile: Determination by HPLC-DAD-MS/MS method. J. Agric. Food Chem. 2012, 60, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Pawlus, A.D.; Sahli, R.; Bisson, J.; Rivière, C.; Delaunay, J.C.; Richard, T.; Gómes, E.; Bordenave, L.; Waffo-Téguo, P.; Mérillon, J.-M. Stilbenoid profiles of canes from Vitis and Muscadinia species. J. Agric. Food Chem. 2013, 61, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Keinanen, M.; Julkunen-Tiitto, R. Effect of sample preparation method on Birch (Betula pendula Roth) leaf phenolics. J. Agric. Food Chem. 1996, 44, 2724–2727. [Google Scholar] [CrossRef]
- De Torres, C.; Diaz-Maroto, M.C.; Hermosin-Gutierrez, I.; Perez-Coello, M.S. Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Anal. Chim. Acta 2010, 660, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, J.V.; García-Alvarado, M.A.; Carcel, J.A.; Mulet, A. Extraction kinetics modeling of antioxidants from grape stalk (Vitis vinifera var. Bobal): Influence of drying conditions. J. Food Eng. 2010, 101, 49–58. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Guerrero, R.F.; Fernández-Marin, M.I.; Cantos-Villar, E.; Palma, M. Ultrasound-assisted extraction of stilbenoids from grape stems. J. Agric. Food Chem. 2013, 61, 12549–12556. [Google Scholar] [CrossRef] [PubMed]
- Karacabey, E.; Mazza, G.; Bayindirli, L.; Artik, N. Extraction of bioactive compounds from milled grape canes (Vitis vinifera) using a pressurized low-polarity extractor. Food Bioprocess Technol. 2009, 5, 359–371. [Google Scholar] [CrossRef]
- Püssa, T.; Floren, J.; Kuldkepp, P.; Raal, A. Survey of grapevine Vitis vinifera stem polyphenols by liquid chromatography-diode array detection-tandem mass spectrometry. J. Agric. Food Chem. 2006, 54, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Schnee, S.; Queiroz, E.F.; Voinesco, F.; Marcourt, L.; Dubuis, P.H.; Wolfender, J.L.; Gindro, K. Vitis vinifera canes, a new source of antifungal compounds against Plasmopara viticola, Erysiphe necator, and Botrytis cinerea. J. Agric. Food Chem. 2013, 61, 5459–5467. [Google Scholar] [CrossRef] [PubMed]
- Houillé, B.; Besseau, S.; Courdavault, V.; Oudin, A.; Glévarec, G.; Delanoue, G.; Guérin, L.; Simkin, A.J.; Papon, N.; Clastre, M.; et al. Biosynthetic origin of E-Resveratrol accumulation in grape canes during postharvest storage. J. Agric. Food Chem. 2015, 63, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Wang, L.; Wu, B.; Li, S. Trans-resveratrol concentrations in seed and berry skin in Vitis evaluated at the germoplasm level. J. Agric. Food Chem. 2006, 54, 8804–8811. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, J.B.; Crespo-Corral, E.; Santos-Delgado, M.J.; Orea, J.M.; Gonzalez-Urena, A. Analysis of trans-resveratrol by laser ionization mass spectrometry and HPLC with fluorescence detection. J. Chrom. A 2005, 1074, 133–138. [Google Scholar]
- Sample Availability: Samples of the extracts are available from the authors.


| Solvent | Temperature | Total Stilbenes (mg kg−1 Dry Weight) |
|---|---|---|
| EtOH | 25 °C | 540.7 ± 11.1 a |
| 80% EtOH in water | 753.2 ± 9.2 b | |
| 60% EtOH in water | 721.9 ± 19.1 b | |
| 80% MeOH in water | 765.4 ± 16.5 b | |
| 60% acetone in water | 831.9 ± 9.2 b | |
| EtOH | 65 °C | 579.1 ± 43.2 a |
| 80% EtOH in water | 633.4 ± 30.0 a | |
| 60% EtOH in water | 689.5 ± 25.0 a | |
| 80% MeOH in water | 551.4 ± 14.0 b | |
| 60% acetone in water | 684.5 ± 28.0 a | |
| EtOH | 75 °C | 732.4 ± 26.0 a |
| 80% EtOH in water | 1365.8 ± 23.0 b | |
| 60% EtOH in water | 1362.9 ± 19.8 b | |
| 80% MeOH in water | 578.7 ± 11.2 c | |
| 60% acetone in water | - |
| Condition | Total Stilbenes (mg·kg−1 Dry Weight) |
|---|---|
| Sample–Solvent Ratio | |
| 1:20 | 450.4 ± 11.0 a |
| 1:25 | 459.7 ± 9.5 a |
| 1:30 | 556.4 ± 11.7 b |
| 1:35 | 677.9 ± 19.9 c |
| 1:40 | 1005.1 ± 38.4 d |
| 1:45 | 998.0 ± 33.2 d |
| 1:50 | 995.1 ± 31.8 d |
| Extraction Time (min) | |
| 5 | 932.1 ± 22.5 a |
| 10 | 1014.5 ± 36.2 b |
| 15 | 997.10 ± 29.2 b |
| 20 | 1008.0 ± 31.5 b |
| 25 | 999.9 ± 28.9 b |
| 30 | 1003.8 ± 37.5 b |
| 35 | 1002.1 ± 44.1 b |
| Precision | trans-Piceid | Piceatannol | trans-Resveratrol | trans-ε-Viniferin |
|---|---|---|---|---|
| Intra-Day (n = 5) | ||||
| Mean (mg·kg–1 dry weight) | 49.2 | 17.8 | 35.3 | 690.4 |
| RSD (%) | 8.8 | 8.9 | 6.2 | 4.4 |
| Inter-Day (n = 5) | ||||
| Mean (mg·kg–1 dry weight) | 49.8 | 18.4 | 34.2 | 725.8 |
| RSD (%) | 10.0 | 9.5 | 4.5 | 4.9 |
| Cultivar | Cane Sample | trans-Piceid a | Piceatannol a | trans-Resveratrol a | trans-ε-Viniferin a | Total Stilbenes a |
|---|---|---|---|---|---|---|
| White | Palomino Fino-1 * | 50.6 ± 1.7 | 18.9 ± 0.6 | 37.5 ± 2.6 | 692.0 ± 5.9 | 799.0 ± 28.3 |
| Sauvignon Blanc | 44.6 ± 0.4 | 28.9 ± 0.6 | 85.8 ± 2.1 | 1921.6 ± 26.7 | 2080.9 ± 73.2 | |
| Palomino fino-2 | 32.3 ± 2.0 | 12.4 ± 0.2 | 48.7 ± 5.6 | 679.0 ± 10.2 | 772.4 ± 31.3 | |
| Melissa | 856.5 ± 20.9 | 160.2 ± 6.0 | 1529.4 ± 43.8 | 620.1 ± 7.8 | 3166.2 ± 108.8 | |
| Victoria | 430.7 ± 10.7 | 53.1± 2.0 | 710.4 ± 20.3 | 860.5 ± 10.9 | 2054.7 ± 62.1 | |
| Matilde | 811.4 ± 19.7 | 89.1 ± 3.4 | 863.2 ± 24.7 | 1421.6 ± 17.9 | 3185.3 ± 98.6 | |
| Average white | 371.0 ± 9.2 | 60.4 ± 2.1 | 545.8 ± 16.5 | 1032.5 ± 13.2 | 2009.7 ± 76.3 | |
| Red | Tempranillo | 77.8 ± 2.8 | 30.2 ± 0.1 | 45.6 ± 1.2 | 807.2 ± 11.1 | 960.8 ± 73.8 |
| Tintilla de Rota | 952.8 ± 23.7 | 66.0 ± 2.7 | 575.1 ± 16.5 | 1964.8 ± 24.8 | 3558.7 ± 81.4 | |
| Vitissylvestris-1 | 30.1 ± 0.3 | 14.6 ± 0.2 | 122.5 ± 1.9 | 797.8 ± 6.6 | 965.0 ± 64.5 | |
| Vitis sylvestris-2 | 43.2 ± 2.5 | 21.0 ± 1.3 | 104.9 ± 10.4 | 1332.5 ± 26.5 | 1501.6 ± 86.2 | |
| Jaen Tinto | 56.0 ± 1.7 | 21.3 ± 2.8 | 174.8 ± 7.7 | 431.4 ± 10.7 | 683.5 ± 68.9 | |
| Rome | 348.4 ± 8.7 | 111.6 ± 4.2 | 107.1 ± 3.1 | 1494.1 ± 18.8 | 2061.2 ± 38.7 | |
| Zinfandel | 319.1 ± 7.6 | 76.0 ± 2.8 | 529.2 ± 15.1 | 2296.1 ± 29.0 | 3220.4 ± 20.5 | |
| Tannat | 502.9 ± 12.9 | 44.2 ± 1.7 | 469.2 ± 13.4 | 2253.1 ± 28.4 | 3269.4 ± 23.9 | |
| Carmenere | 181.6 ± 4.3 | 37.3 ± 1.4 | 242.8 ± 6.9 | 973.1 ± 12.3 | 1434.8 ± 38.9 | |
| Malbec | 353.2 ± 8.5 | 76.5 ± 2.5 | 664.9 ± 19.0 | 2253.9 ± 28.4 | 3348.5 ± 61.6 | |
| Flame seedless | 199.1 ± 4.9 | 90.6 ± 3.2 | 598.4 ± 17.1 | 1715.6 ± 21.6 | 2603.7 ± 70.4 | |
| Crimson seedless | 419.4 ± 10.3 | 30.9 ± 1.2 | 237.5 ± 6.8 | 1587.8 ± 20.0 | 2275.6 ± 86.7 | |
| Red Globe | 391.3 ± 9.5 | 40.4 ± 1.5 | 389.7 ± 11.2 | 1784.7 ± 22.5 | 2606.1 ± 51.3 | |
| Moscatel rosado | 747.7 ± 18.2 | 67.6 ± 2.6 | 966.8 ± 27.7 | 1051.2 ± 13.3 | 2833.3 ± 66.1 | |
| Average red | 267.2 ± 8.3 | 57.7 ± 2.0 | 467.3 ± 11.3 | 1712.2 ± 19.6 | 2237.3 ± 62.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñeiro, Z.; Marrufo-Curtido, A.; Serrano, M.J.; Palma, M. Ultrasound-Assisted Extraction of Stilbenes from Grape Canes. Molecules 2016, 21, 784. https://doi.org/10.3390/molecules21060784
Piñeiro Z, Marrufo-Curtido A, Serrano MJ, Palma M. Ultrasound-Assisted Extraction of Stilbenes from Grape Canes. Molecules. 2016; 21(6):784. https://doi.org/10.3390/molecules21060784
Chicago/Turabian StylePiñeiro, Zulema, Almudena Marrufo-Curtido, Maria Jose Serrano, and Miguel Palma. 2016. "Ultrasound-Assisted Extraction of Stilbenes from Grape Canes" Molecules 21, no. 6: 784. https://doi.org/10.3390/molecules21060784
APA StylePiñeiro, Z., Marrufo-Curtido, A., Serrano, M. J., & Palma, M. (2016). Ultrasound-Assisted Extraction of Stilbenes from Grape Canes. Molecules, 21(6), 784. https://doi.org/10.3390/molecules21060784