Anti-Inflammatory Effects of Agrimoniin-Enriched Fractions of Potentilla erecta
Abstract
:1. Introduction
2. Results
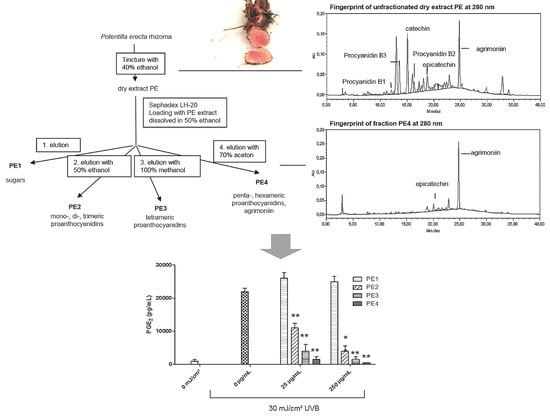
2.1. Fractionation of PE Extract
2.2. Inhibition of UVB- Induced COX-2 Expression and PGE2-Production
2.3. Inhibition of EGF-Induced EGFR Phosphorylation
2.4. Inhibition of UVB-Induced Erythema in Vivo
3. Discussion
4. Experimental Section
4.1. Extracts and Chemicals
4.2. Cell Culture
4.3. PGE2-ELISA
4.4. Suction Blister Experiments
4.5. Western Blot
4.6. Immunocytochemistry
4.7. Cell Death Assay
4.8. UV Erythema Test
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomczyk, M.; Latté, K.P. Potentilla—A review of its phytochemical and pharmacological profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Ditfurth, A.V.; Amann, F.; Güthlin, C.; Rostock, M.; Trittler, R.; Kümmerer, K.; Merfort, I. Tormentil for active ulcerative colitis: An open-label, dose-escalating study. J. Clin. Gastroenterol. 2007, 41, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Kanoh, R.; Hatano, T.; Ito, H.; Yoshida, T.; Akagi, M. Effects of tannins and related polyphenols on superoxide-induced histamine release from rat peritoneal mast cells. Phytomedicine 2000, 7, 297–302. [Google Scholar] [CrossRef]
- Lund, K. Tormentillwurzelstock, Phytochemische Untersuchungen des Rhizoms von Potentilla erecta (L.) Räuschel. Ph.D. Thesis, University of Freiburg, Freiburg, Germany, 1986. [Google Scholar]
- Bos, M.A.; Vennat, B.; Meunier, M.T.; Pouget, M.P.; Pourrat, A.; Fialip, J. Procyanidins from tormentil: Antioxidant properties towards lipoperoxidation and anti-elastase activity. Biol. Pharm. Bull. 1996, 19, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Vennat, B.; Bos, M.A.; Pouget, M.P.; Pourrat, A. Potentilla tormentilla: Standardisation d’un extrait hydrosoluble, controles et applications. J. Pharm. Belg. 1994, 49, 5–11. [Google Scholar]
- Fecka, I.; Kucharska, A.Z.; Kowalczyk, A. Quantification of tannins and related polyphenols in commercial products of tormentil (Potentilla tormentilla). Phytochem. Anal. PCA 2015, 26, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Ashida, M.; Bito, T.; Budiyanto, A.; Ichihashi, M.; Ueda, M. Involvement of EGF receptor activation in the induction of cyclooxygenase-2 in HaCaT keratinocytes after UVB. Exp. Dermatol. 2003, 12, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Sachsenmaier, C.; Radler-Pohl, A.; Zinck, R.; Nordheim, A.; Herrlich, P.; Rahmsdorf, H.J. Involvement of growth factor receptors in the mammalian UVC response. Cell 1994, 78, 963–972. [Google Scholar] [CrossRef]
- Hsu, S. Green tea and the skin. J. Am. Acad. Dermatol. 2005, 52, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Tunón, H.; Olavsdotter, C.; Bohlin, L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef]
- Tomczyk, M.; Leszczyńska, K.; Jakoniuk, P. Antimicrobial activity of Potentilla species. Fitoterapia 2008, 79, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R.; Wiater, A.; Locatelli, M.; Pleszczyńska, M.; Tomczyk, M. Aqueous Extracts of Selected Potentilla Species Modulate Biological Activity of Human Normal Colon Cells. Curr. Drug Targets 2014, 16, 1495–1502. [Google Scholar] [CrossRef]
- Tomczyk, M.; Wiater, A.; Pleszczyńska, M. In vitro anticariogenic effects of aerial parts of Potentilla recta and its phytochemical profile. Phytother. Res. PTR 2011, 25, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Tomovic, M.T.; Cupara, S.M.; Popovic-Milenkovic, M.T.; Ljujic, B.T.; Kostic, M.J.; Jankovic, S.M. Antioxidant and anti-inflammatory activity of Potentilla reptans L. Acta Pol. Pharm. 2015, 72, 137–145. [Google Scholar] [PubMed]
- Bazylko, A.; Piwowarski, J.P.; Filipek, A.; Bonarewicz, J.; Tomczyk, M. In vitro antioxidant and anti-inflammatory activities of extracts from Potentilla recta and its main ellagitannin, agrimoniin. J. Ethnopharmacol. 2013, 149, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.L.; Thomas, G.; Barbosa Filho, J.M. Anti-inflammatory actions of tannins isolated from the bark of Anacardium occidentale L. J. Ethnopharmacol. 1985, 13, 289–300. [Google Scholar] [PubMed]
- Geiger, C.; Scholz, E.; Rimpler, H. Ellagitannins from Alchemilla xanthochlora and Potentilla erecta*. Planta Med. 1994, 60, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Pilipović, S.; Bosnić, T.; Redžić, S.; Mijanović, M. Topical anti-inflammatory effect of acetone rhizome/root extract of Potentilla malýana Borbas. Planta Med. 2007, 73. [Google Scholar] [CrossRef]
- Hrenn, A.; Steinbrecher, T.; Labahn, A.; Schwager, J.; Schempp, C.M.; Merfort, I. Plant phenolics inhibit neutrophil elastase. Planta Med. 2006, 72, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Wiedow, O.; Wiese, F.; Christophers, E. Lesional elastase activity in psoriasis. Diagnostic and prognostic significance. Arch. Dermatol. Res. 1995, 287, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, H. Neutrophil elastase inhibitors as treatment for COPD. Expert Opin. Investig. Drugs 2002, 11, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, W.; Liu, Y.; Remmer, H.A.; Voorhees, J.; Fisher, G.J. Solar ultraviolet irradiation induces decorin degradation in human skin likely via neutrophil elastase. PLoS ONE 2013, 8, e72563. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, A.J.; Lippman, S.M.; Mann, J.R.; Subbaramaiah, K.; DuBois, R.N. Cyclooxygenase-2 and epidermal growth factor receptor: Pharmacologic targets for chemoprevention. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Oxidative inhibition of receptor-type protein-tyrosine phosphatase kappa by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. J. Biol. Chem. 2006, 281, 27389–27397. [Google Scholar] [CrossRef] [PubMed]
- Knebel, A.; Rahmsdorf, H.J.; Ullrich, A.; Herrlich, P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 1996, 15, 5314–5325. [Google Scholar] [PubMed]
- Sheng, H.; Shao, J.; Washington, M.K.; DuBois, R.N. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J. Biol. Chem. 2001, 276, 18075–18081. [Google Scholar] [CrossRef] [PubMed]
- Vennat, B.; Pouget, M.P.; Pourrat, A.; Pourrat, H. Proanthocyanidines: Composition qualitatives et quantitative d’un extrait de rhizomes de Potentilla tormentilla (Rosacées). J. Pharm. Belg. 1992, 47, 485–493. [Google Scholar]
- Schempp, C.M.; Winghofer, B.; Lüdtke, R.; Simon-Haarhaus, B.; Schöpf, E.; Simon, J.C. Topical application of St John’s wort (Hypericum perforatum L.) and of its metabolite hyperforin inhibits the allostimulatory capacity of epidermal cells. Br. J. Dermatol. 2000, 142, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the PE fractions are not available from the authors.









© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, J.; Casetti, F.; Bullerkotte, U.; Haarhaus, B.; Vagedes, J.; Schempp, C.M.; Wölfle, U. Anti-Inflammatory Effects of Agrimoniin-Enriched Fractions of Potentilla erecta. Molecules 2016, 21, 792. https://doi.org/10.3390/molecules21060792
Hoffmann J, Casetti F, Bullerkotte U, Haarhaus B, Vagedes J, Schempp CM, Wölfle U. Anti-Inflammatory Effects of Agrimoniin-Enriched Fractions of Potentilla erecta. Molecules. 2016; 21(6):792. https://doi.org/10.3390/molecules21060792
Chicago/Turabian StyleHoffmann, Julia, Federica Casetti, Ute Bullerkotte, Birgit Haarhaus, Jan Vagedes, Christoph M. Schempp, and Ute Wölfle. 2016. "Anti-Inflammatory Effects of Agrimoniin-Enriched Fractions of Potentilla erecta" Molecules 21, no. 6: 792. https://doi.org/10.3390/molecules21060792
APA StyleHoffmann, J., Casetti, F., Bullerkotte, U., Haarhaus, B., Vagedes, J., Schempp, C. M., & Wölfle, U. (2016). Anti-Inflammatory Effects of Agrimoniin-Enriched Fractions of Potentilla erecta. Molecules, 21(6), 792. https://doi.org/10.3390/molecules21060792