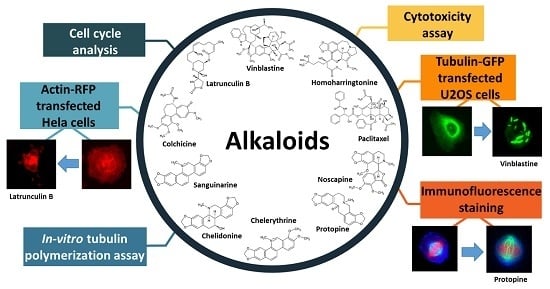
The Interference of Selected Cytotoxic Alkaloids with the Cytoskeleton: An Insight into Their Modes of Action
Abstract
:1. Introduction
2. Results
2.1. Anti-Proliferative Activity of Alkaloids
2.2. Do Alkaloids Interfere with Microtubules in Living Cells?
2.2.1. Influence on Microtubules
2.2.2. Influence on Spindle Apparatus
2.3. Benzophenanthridine Alkaloids Inhibited Tubulin Polymerization in Vitro
2.4. Influence of Benzophenanthridine Alkaloids on Actin Filaments in Hela Cells
2.5. Cell Cycle Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cytotoxicity Assay
4.4. Imaging of Tubulin-GFP Transfected U2OS Cells
4.5. Immunofluorescence Staining
4.6. Imaging of Actin-RFP Transfected Hela Cells
4.7. In-Vitro Tubulin Polymerization Assay
4.8. Cell Cycle Analysis
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wink, M. Molecular modes of action of cytotoxic alkaloids: From DNA intercalation, spindle poisoning, topoisomerase inhibition to apoptosis and multiple drug resistance. Alkaloids Chem. Biol. 2007, 64. [Google Scholar] [CrossRef]
- Wink, M. Introduction. In Annual Plant Reviews: Functions and Biotechnology of Plant Secondary Metabolites, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2010; Volume 39, pp. 1–20. [Google Scholar]
- Lu, J.J.; Bao, J.L.; Chen, X.P.; Huang, M.; Wang, Y.T. Alkaloids isolated from natural herbs as the anticancer agents. Evid. Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Sun, H.; Zhang, A.-H.; Xu, H.-Y.; Yan, G.-L.; Han, Y.; Wang, X.-J. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar] [CrossRef]
- Kittakoop, P.; Mahidol, C.; Ruchirawat, S. Alkaloids as important scaffolds in therapeutic drugs for the treatments of cancer, tuberculosis, and smoking cessation. Curr. Top. Med. Chem. 2014, 14, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Schimmer, O. Molecular modes of action of defensive secondary metabolites. In Annual Plant Reviews: Functions and Biotechnology of Plant Secondary Metabolites, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2010; Volume 39, pp. 21–161. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2014. [Google Scholar]
- Fletcher, D.A.; Mullins, D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wickstead, B.; Gull, K. The evolution of the cytoskeleton. J. Cell. Biol. 2011, 194, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Petrasek, J.; Schwarzerova, K. Actin and microtubule cytoskeleton interactions. Curr. Opin. Plant. Biol. 2009, 12, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, G.; Gill, R.K.; Soni, R.; Bariwal, J. Recent developments in tubulin polymerization inhibitors: An overview. Eur. J. Med. Chem. 2014, 87, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Field, J.J.; Kanakkanthara, A.; Miller, J.H. Microtubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule function. Bioorg. Med. Chem. 2014, 22, 5050–5059. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A. Effects of cytochalasin and phalloidin on actin. J. Cell. Biol. 1987, 105, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tanaka, M.; Krstin, S.; Peixoto, H.S.; Moura, C.C.D.M.; Wink, M. Cytoskeletal interference―A new mode of action for the anticancer drugs camptothecin and topotecan. Eur. J. Pharmacol. 2016. submitted. [Google Scholar]
- Slaninová, I.; Pěnčíková, K.; Urbanová, J.; Slanina, J.; Táborská, E. Antitumour activities of sanguinarine and related alkaloids. Phytochem. Rev. 2013, 13, 51–68. [Google Scholar] [CrossRef]
- Basu, P.; Bhowmik, D.; Kumar, G.S. The benzophenanthridine alkaloid chelerythrine binds to DNA by intercalation: Photophysical aspects and thermodynamic results of iminium versus alkanolamine interaction. J. Photoch. Photobiol. B 2013, 129, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Kamei, J.; Iwamoto, Y.; Misawa, M.; Kasuya, Y. Effects of rimcazole, a specific antagonist of sigma sites, on the antitussive effects of non-narcotic antitussive drugs. Eur. J. Pharmacol. 1993, 242, 209–211. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Mojaverian, N. Efffect of noscapine, the antitussive opioid alkaloid, on bradykinin-induced smooth muscle contraction in the isolated ileum of the guinea-pig. Acta. Physiol. Hung. 2001, 88, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.N.; Wu, T.S.; Lu, S.T.; Wu, Y.C.; Huang, T.F.; Teng, C.M. Ca2+-channel blockade in rat thoracic aorta by protopine isolated from Corydalis tubers. Jpn. J. Pharmacol. 1992, 58. [Google Scholar] [CrossRef]
- Lü, S.Q.; Wang, J.M. Homoharringtonine and omacetaxine for myeloid hematological malignancies. J. Hematol. Oncol. 2014, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grintsevich, E.E.; Reisler, E. Cytoskeleton dynamics and binding factors. In The Cytoskeleton: Imaging, Isolation, and Interaction; Dermietzel, R., Ed.; Humana Press: New York, NY, USA, 2013; pp. 63–83. [Google Scholar]
- Margolis, R.L.; Wilson, L. Microtubule treadmilling: What goes around comes around. Bioessays 1998, 20, 830–836. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Graves, P.R.; Thoma, R.S.; Wu, Z.; Shaw, A.S.; Piwnica-Worms, H. Mitotic and G2 checkpoint control: Regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 1997, 277, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.; Nurse, P. Cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell 1986, 45, 145–153. [Google Scholar] [CrossRef]
- Rupes, I.; Webb, B.A.; Mak, A.; Young, P.G. G2/M arrest caused by actin disruption is a manifestation of the cell size checkpoint in fission yeast. Mol. Biol. Cell 2001, 12, 3892–3903. [Google Scholar] [CrossRef] [PubMed]
- Schiff, P.B.; Fant, J.; Horwitz, S.B. Promotion of microtubule assembly in vitro by taxol. Nature 1979, 277, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N. Taxol-induced polymerization of purified tubulin. Mechanism of action. J. Biol. Chem. 1981, 256, 10435–10441. [Google Scholar] [PubMed]
- Schiff, P.B.; Horwitz, S.B. Taxol assembles tubulin in the absence of exogenous guanosine 5′-triphosphate or microtubule-associated proteins. Biochemistry 1981, 20, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.; Knipling, L. Antimicrotubule properties of benzophenanthridine alkaloids. Biochemistry 1993, 32, 13334–13339. [Google Scholar] [CrossRef] [PubMed]
- Panzer, A.; Joubert, A.M.; Bianchi, P.C.; Hamel, E.; Seegers, J.C. The effects of chelidonine on tubulin polymerisation, cell cycle progression and selected signal transmission pathways. Eur. J. Cell. Biol. 2001, 80, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lopus, M.; Panda, D. The benzophenanthridine alkaloid sanguinarine perturbs microtubule assembly dynamics through tubulin binding. A possible mechanism for its antiproliferative activity. FEBS J. 2006, 273, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Gupta, S.; Husain, M.M.; Heiskanen, K.M.; Mukhtar, H. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin. Cancer Res. 2000, 6, 1524–1528. [Google Scholar] [PubMed]
- Schmeller, T.; Latz-Bruning, B.; Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry 1997, 44, 257–266. [Google Scholar] [CrossRef]
- Britto, P.J.; Knipling, L.; McPhie, P.; Wolff, J. Thiol-disulphide interchange in tubulin: Kinetics and the effect on polymerization. Biochem. J. 2005, 389, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, R.; Sakai, H. Role of tubulin -SH groups in polymerization to microtubules: Functional -SH groups in tubulin for polymerization. J. Biochem. 1974, 76, 651–654. [Google Scholar] [PubMed]
- Malikova, J.; Zdarilova, A.; Hlobilkova, A. Effects of sanguinarine and chelerythrine on the cell cycle and apoptosis. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2006, 150, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Peng, L.; Liu, J.J.; Huang, Q.Y.; Peng, F.; Yuan, S.S. Identification of differentially expressed proteins in the liver of Oncomelania snails induced by Eomecon chinanthe sanguinarine. Zhonghua Yu Fang Yi Xue Za Zhi 2010, 44, 490–493. [Google Scholar] [PubMed]
- Mozhenok, T.P.; Beliaeva, T.N.; Leont'eva, E.A.; Faddeeva, M.D. Effect of alkaloid sanguinarine and a pharmaceutical preparation ukrain on modulation of vesicular membrane fusion and actin cytoskeleton of macrophages. Tsitologiia 2005, 47, 860–865. [Google Scholar] [PubMed]
- Kim, O.; Hwangbo, C.; Kim, J.; Li, D.H.; Min, B.S.; Lee, J.H. Chelidonine suppresses migration and invasion of MDA-MB-231 cells by inhibiting formation of the integrin-linked kinase/PINCH/α-parvin complex. Mol. Med. Rep. 2015, 12, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Landen, J.W.; Lang, R.; McMahon, S.J.; Rusan, N.M.; Yvon, A.M.; Adams, A.W.; Sorcinelli, M.D.; Campbell, R.; Bonaccorsi, P.; Ansel, J.C.; et al. Noscapine alters microtubule dynamics in living cells and inhibits the progression of melanoma. Cancer Res. 2002, 62, 4109–4114. [Google Scholar] [PubMed]
- Zhou, J.; Panda, D.; Landen, J.W.; Wilson, L.; Joshi, H.C. Minor alteration of microtubule dynamics causes loss of tension across kinetochore pairs and activates the spindle checkpoint. J. Biol. Chem. 2002, 277, 17200–17208. [Google Scholar] [CrossRef] [PubMed]
- Checchi, P.M.; Nettles, J.H.; Zhou, J.; Snyder, J.P.; Joshi, H.C. Microtubule-interacting drugs for cancer treatment. Trends Pharmacol. Sci. 2003, 24, 361–365. [Google Scholar] [CrossRef]
- Ke, Y.; Ye, K.; Grossniklaus, H.E.; Archer, D.R.; Joshi, H.C.; Kapp, J.A. Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunol. Immun. 2000, 49, 217–225. [Google Scholar] [CrossRef]
- Chen, C.H.; Liao, C.H.; Chang, Y.L.; Guh, J.H.; Pan, S.L.; Teng, C.M. Protopine, a novel microtubule-stabilizing agent, causes mitotic arrest and apoptotic cell death in human hormone-refractory prostate cancer cell lines. Cancer Lett. 2012, 315. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, V.; Gutman, J.A.; Pollyea, D.A.; Jimeno, A. Omacetaxine mepesuccinate for the treatment of chronic myeloid leukemia. Drugs Today 2013, 49, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Nurcahyanti, A.D.R.; Wink, M. Cytotoxic potentiation of vinblastine and paclitaxel by l-canavanine in human cervical cancer and hepatocellular carcinoma cells. Phytomedicine 2015, 22, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Gell, C.; Friel, C.T.; Borgonovo, B.; Drechsel, D.N.; Hyman, A.A.; Howard, J. Purification of tubulin from porcine brain. In Microtubule Dynamics: Methods and protocols; Straube, A., Ed.; Humana Press: New York, NY, USA, 2011; pp. 15–28. [Google Scholar]
- Su, S.; Cheng, X.; Wink, M. Cytotoxicity of arctigenin and matairesinol against the t-cell lymphoma cell line ccrf-cem. J. Pharm. Pharmacol. 2015, 67, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Colchicine, vinblastine, sanguinarine, chelerythrine, chelidonine, protopine, noscapine and homoharingtonine are commercially available.











| Compounds | IC50 | ||
|---|---|---|---|
| Hela | MCF-7 | U2OS | |
| Colchicine * | 0.01 ± 0.01 μM | 0.02 ± 0.01 μM | 0.01 ± 0.01 μM |
| Vinblastine * | 0.02 ± 0.01 nM | 0.07 ± 0.05 nM | 0.10 ± 0.05 nM |
| Paclitaxel * | 0.08 ± 0.10 nM | 1.05 ± 0.43 μM | 0.23 ± 0.10 μM |
| Latrunculin B ** | 7.13 ± 0.61 μM | 36.78 ± 2.25 μM | 5.48 ± 0.68 μM |
| Sanguinarine | 1.03 ± 0.04 μM | 1.24 ± 0.21 μM | 0.92 ± 0.53 μM |
| Chelerythrine | 3.85 ± 2.10 μM | 7.63 ± 3.43 μM | 2.88 ± 0.76 μM |
| Chelidonine | 1.13 ± 0.37 μM | 3.85 ± 0.10 μM | 3.86 ± 1.99 μM |
| Protopine | 27.50 ± 11.77 μM | 67.28 ± 9.06 μM | 62.36 ± 9.90 μM |
| Noscapine | 16.81 ± 9.79 μM | 35.20 ± 8.18 μM | 60.85 ± 12.17 μM |
| Homoharringtonine | 9.03 ± 0.38 nM | 0.87 ± 0.45 nM | 3.0 ± 1.67 nM |
| Compounds | IC50 |
|---|---|
| Colchicine | 2.89 ± 0.36 µM |
| Vinblastine | 1.42 ± 0.05 µM |
| Paclitaxel | 38.19 ± 3.33 μM |
| Latrunculin B | >1 mM |
| Sanguinarine | 48.41 ± 3.73 μM |
| Chelerythrine | 206.39 ± 4.20 μM |
| Chelidonine | 34.51 ± 9.47 μM |
| Protopine | >1 mM |
| Noscapine | >1 mM |
| Homoharringtonine | >1 mM |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tanaka, M.; Krstin, S.; Peixoto, H.S.; Wink, M. The Interference of Selected Cytotoxic Alkaloids with the Cytoskeleton: An Insight into Their Modes of Action. Molecules 2016, 21, 906. https://doi.org/10.3390/molecules21070906
Wang X, Tanaka M, Krstin S, Peixoto HS, Wink M. The Interference of Selected Cytotoxic Alkaloids with the Cytoskeleton: An Insight into Their Modes of Action. Molecules. 2016; 21(7):906. https://doi.org/10.3390/molecules21070906
Chicago/Turabian StyleWang, Xiaojuan, Mine Tanaka, Sonja Krstin, Herbenya Silva Peixoto, and Michael Wink. 2016. "The Interference of Selected Cytotoxic Alkaloids with the Cytoskeleton: An Insight into Their Modes of Action" Molecules 21, no. 7: 906. https://doi.org/10.3390/molecules21070906
APA StyleWang, X., Tanaka, M., Krstin, S., Peixoto, H. S., & Wink, M. (2016). The Interference of Selected Cytotoxic Alkaloids with the Cytoskeleton: An Insight into Their Modes of Action. Molecules, 21(7), 906. https://doi.org/10.3390/molecules21070906