Assessment of Phenolic Compounds and Anti-Inflammatory Activity of Ethyl Acetate Phase of Anacardium occidentale L. Bark
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Validation and Quantitation of Compounds
2.3. Anti-Inflammatory Activity
2.3.1. Effect of EtOAc Phases on Carrageenan, Bradykinin and Prostaglandin-induced Mice Paw Edema
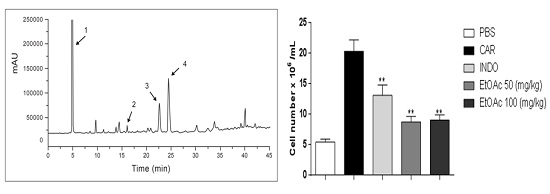
2.3.2. Decrease in Carrageenan-Induced Peritonitis by the EtOAc Phase
2.3.3. Effect of EtOAc Phases on Leukocyte Subsets in pPeritoneum
2.3.4. Effect of EtOAc Phase on IL-1β, IL-6, IL-10 and TNF-α
3. Materials and Methods
3.1. Plant Material
3.2. Extract Preparation
3.3. Method Development
3.4. Identification of Compounds
3.5. Validation
3.6. Anti-Inflammatory Activity
3.6.1. Animals
3.6.2. Inflammatory Paw Edema
3.6.3. Peritoneal Inflammation Model
3.6.4. Measurement of Cytokine Levels by ELISA
3.6.5. Phenotyping of Leukocytes
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silva-Luz, C.L.; Pirani, J.R. Anacardiaceae. In Catálogo de Plantas e Fungos do Brasil; Forzza, R.C., Baumgratz, J.F.A., Bicudo, C.E.M., Carvalho, A.A., Jr., Costa, A., Costa, D.P., Hopkins, M., Leitman, P.M., Lohmann, L.G., Maia, L.C., et al., Eds.; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2010; Volume 1, pp. 599–602. [Google Scholar]
- Mazzetto, S.L.; Lomonaco, D.; Mele, G. Óleo da castanha de caju: Oportunidades e desafios no contexto do desenvolvimento e sustentabilidade industrial. Quim. Nova 2009, 32, 732–741. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Plantas de interesse ao SUS. 2009. Available online: http://portal.saude.gov.br/portal/saude/profissional/visualizar_texto.cfm?idtxt=30 277&janela=1 (accessed on 26 November 2014).
- Mota, M.L.R.; Thomas, G.; Barbosa Filho, J.M. Anti-inflamatory actions of tannins isolated from the bark of Anacardium occidentale L. J. Ethnopharmacol. 1985, 13, 289–300. [Google Scholar] [PubMed]
- Vanderlinde, F.A.; Landim, H.F.; Costa, E.A.; Galdino, P.M.; Marciel, M.A.M.; Anjos, G.C.; Malvar, D.C.; Cortes, W.S.; Rocha, F.F. Evaluation of the antinociceptive and anti-inflammatory effects of the acetone extract from Anacardium occidentale L. Braz. J. Pharm. Sci. 2009, 45, 437–442. [Google Scholar] [CrossRef]
- Carvalho, M.G. Desenvolvimento e validação de um Novo Método Para Análise de Compostos Fenólicos em Produtos Contendo Schinus Terebinthifolius Raddi (Anarcardiaceae) por CLAE/DAD; Dissertação de Mestrado, Universidade Federal do Rio Grande do Norte: Natal, Rio Grande do Norte, Brasil, 25 February 2011. [Google Scholar]
- Sokeng, S.D.; Kamtchouing, P.; Watcho, P.; Jatsa, H.B.; Moundipa, P.F.; Ngounou, F.N.; Lontsi, D.; Bopelet, M. Hypoglycemic activity of Anacardium occidentale L. aqueous extract in normal and streptozotocin-induced diabetic rats. Diabetes Res. 2001, 36, 1–9. [Google Scholar]
- Barbosa-Filho, J.M.; Vasconcelos, T.H.C.; Alencar, A.A.; Batista, L.M.; Oliveira, R.A.G.; Guedes, D.N.; Falcão, H.S.; Moura, M.D.; Diniz, M.F.F.M.; Modesto-Filho, J. Plants and their active constituents from South, Central, and North America with hypoglycemic activity. Rev. Bras. Farmacogn. 2005, 15, 392–413. [Google Scholar] [CrossRef]
- Akinpelu, D.A. Antimicrobial activity of Anacardium occidentale bark. Fitoterapia 2001, 72, 286–287. [Google Scholar] [CrossRef]
- Trevisan, M.T.S.; Pfundstein, B.; Haubner, R.; Würtele, G.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem. Toxicol. 2006, 44, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Konan, N.A.; Bacchi, E.M. Antiulcerogenic effect and acute toxicity of a hydroethanolic extract from the cashew (Anacardium occidentale L.) leaves. J. Ethnopharmacol. 2007, 112, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Ushanandini, S.; Nagaraju, S.; Nayaka, S.C.; Kumar, K.H.; Kemparaju, K.; Girish, K.S. The anti-ophidian properties of Anacardium occidentale bark extract. Immunopharmacol. Immunotoxicol. 2009, 31, 607–615. [Google Scholar] [CrossRef] [PubMed]
- França, F.; Cuba, C.A.; Moreira, E.A.; Almeida, M.; Virgens, M.L.; Marsden, P.D. Avaliação do efeito do extrato de casca de cajueiro-branco (Anacardium occidentales) sobre a infecção por Leishmania (Viannia) braziliensis. Rev. Soc. Bras. Med. Trop. 1993, 26, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Filho, J.M.; Medeiros, K.C.P.; Diniz, M.F.F.M.; Batista, L.M.; Athayde-Filho, P.F.; Silva, M.S.; Cunha, E.V.L.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Natural products inhibitors of the enzyme acetylcholinesterase. Rev. Bras. Farmacogn. 2006, 16, 258–285. [Google Scholar] [CrossRef]
- Olajide, O.A.; Aderogba, M.A.; Adedapo, A.D.; Makinde, J.M. Effects of Anacardium occidentale stem bark extract on in vivo inflammatory models. J. Ethnopharmacol. 2004, 95, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.H.; Citó, A.M.; Lopes, J.A.D.; Costa, D.A.C.; Oliveira, C.A.A.; Costa, A.F.; Brito-Júnior, F.E.M. Fenóis totais, atividade antioxidantes e constituintes químicos de extratos de Anacardium occidentale L., Anacardiaceae. Rev. Bras. Farmacogn. 2010, 20, 106–112. [Google Scholar] [CrossRef]
- Fujita, G.T. BDRA-26-Caju, intenso caju. Rev. Terra da Gente 2008, 5, 42–44. [Google Scholar]
- United States Pharmacopeial Convention. The United States Pharmacopeia; The United States Pharmacopeial Convention: Rockville, MD, USA, 2007. [Google Scholar]
- Lanças, F.M. Cromatografia Líquida Moderna; Átomo, Ed.; Sesccampinas: Campinas, Brazil, 2009; p. 384. [Google Scholar]
- ANVISA BRASIL. Resolução RE No 899, de 29 de maio de 2003. Guia Para a Validação de Métodos Analíticos e Bioanalíticos; Diário Oficial da União: Brasília, DF, Brazil, 2 June 2003.
- Kulkarni, J.M.; Viswanatha Swamy, A.H.M. Cardioprotective effect of gallic acid against doxorubicin-induced myocardial toxicity in albino rats. Indian J. Med. Sci. 2015, 8, 28–35. [Google Scholar]
- Priscilla, D.H.; Prince, P. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem. Biol. Interact. 2009, 179, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kroes, B.H.; van den Berg, A.J.; Quarles van Ufford, H.C.; van Dijk, H.; Labadie, R.P. Anti-inflammatory activity of gallic acid. Planta Med. 1992, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Mejía, E.G.; Chandra, S.; Ramírez-Mares, M.V.; Wang, W. Catalytic inhibition of human DNA topoisomerase by phenolic compounds in Ardisia compressa extracts and their effect on human colon cancer cells. Food Chem. Toxicol. 2006, 44, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.H.; Li, B.L.; Li, N.B.; Luo, H.Q. Sensitive detection of gallic acid based on polyethyleneimine-functionalized graphene modified glassy carbon electrode. Sens. Actuators B 2013, 186, 84–89. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Yang, N. Flow injection chemiluminescent detection of gallic acid in olive fruits. Food Chem. 2007, 105, 340–345. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Chimnoi, N.; Techasakul, S.; Supaphol, P. Gallic acid-loaded electrospun poly(L-lactic acid) fiber mats and their release characteristic. Macromol. Chem. Phys. 2009, 210, 814–822. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zeinegallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.; Filippin-Monteiro, F.B.; Creczynski-Pasa, T.B. Alkyl esters of gallic acid as anticancer agents: A review. Eur. J. Med. Chem. 2013, 60, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Punithavathi, V.R.; Prince, P.S.M.; Kumar, R.; Selvakumari, J. Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur. J. Pharmacol. 2011, 650, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NFkappaB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.C.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [PubMed]
- Odontuya, G.; Hoult, J.R.; Houghton, P.J. Structure-activity relationship for anti-inflammatory effect of luteolin and its derived glycosides. Phytother. Res. 2005, 19, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective role of ternatin anthocyanins and quercetin glycosides from butterfly pea (Clitoria ternatea Leguminosae) blue flower petals against lipopolysaccharide (LPS)-induced inflammation in macrophage cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 1, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Bang, W.Y.; Nair, V.; Angulo-Escalante, M.A.; Cisneros-Zevallos, L.; Heredia, J.B. Protective Role of Flavonoids and Lipophilic Compounds from Jatropha platyphylla on the Suppression of Lipopolysaccharide (LPS)-Induced Inflammation in Macrophage Cells. J. Agric. Food Chem. 2016, 9, 1899–1909. [Google Scholar]
- Doherty, N.S.; Poubelle, P.; Borgeat, P.; Beaver, T.H.; Westrich, G.L.; Schrader, N.L. Intraperitoneal injection of zymosan in mice induces pain, inflammation and the synthesis of peptidoleukotrienes and prostaglandin E2. Prostaglandins 1985, 30, 769–789. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Goldys, A.; Kozakiewicz, E.; Lelito, M.; Plytycz, B.; Van Rooijen, N.; Arnold, B. Resident peritoneal macrophages and mast cells are important cellular sites of COX-1 and COX-2. Arch. Immunol. Ther. Exp. 2009, 57, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.L.; White, G.E.; Greaves, D.R. Chapter. Zymosan induced peritonitis as a simple experimental system for the study of inflammation. Meth. Enzymol. 2009, 461, 379–396. [Google Scholar] [PubMed]
- Ajuebor, M.N.; Virág, L.; Flower, R.J.; Perretti, M.; Szabó, C. Role of inducible nitric oxide synthase in the regulation of neutrophil migration in zymosan-induced inflammation. Immunology 1998, 95, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Ianaro, A.; O’donnell, C.A.; Di Rosa, M.; Liew, F.Y. A nitric oxide synthase inhibitor reduces inflammation, down-regulates inflammatory cytokines and enhances interleukin-10 production in carrageenin-induced oedema in mice. Immunology 1994, 82, 370–375. [Google Scholar] [PubMed]
- Halici, Z.; Dengiz, G.O.; Odabasoglu, F.; Suleyman, H.; Cadirci, E.; Halici, M. Amiodarone has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced paw edema. Eur. J. Pharmacol. 2007, 566, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.E.; Cannavacciulo, R.; Cravatt, B.F.; Martin, B.F. Evaluation of fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology 2008, 54, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, J.P.; Lovato, S.J.; Erion, M.D.; Jeng, A.Y. Modulation of carrageenan-induced hind paw edema by substance P. Inflammation 1994, 18, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.S.; Antonio, M.A. Oral anti-inflammatory and anti-ulcerogenic activities of a hydroalcoholic extract and partitioned fractions of Turnera ulmifolia (Turneraceae). J. Ethnopharmacol. 1998, 61, 215–228. [Google Scholar]
- Loram, L.C.; Fuller, A.; Fick, L.G.; Cartmell, T.; Poole, S.; Mitchell, D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J. Pain 2007, 8, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Mizgerd, J.P.; Spieker, M.R.; Doerschuk, C.K. Early response cytokines and innate immunity: Essential roles for TNF receptor 1 and type 1 IL-1 receptor during Escherichia coli pneumonia in mice. J. Immunol. 2001, 166, 4042–4048. [Google Scholar] [CrossRef] [PubMed]
- Falcão, H.S.; Lima, I.O.; Santos, V.L.; Dantas, H.F.; Diniz, M.F.F.M.; Barbosa-Filho, J.M. Review od the plants with anti-inflammatory activity studied in Brazil. Rev. Bras. Farmacogn 2005, 15, 381–391. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Colell, A.; Mari, M.; Morales, A.; Fernádez-Checa, J.C. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J. Biol. Chem. 1997, 272, 11369–11377. [Google Scholar] [PubMed]
- Shahrzad, S.; Bitsch, I. Determination of gallic acid and its metabolites in human plasma and urine by high-performance liquid chromatography. J. Chromatogr. B 1998, 70, 87–95. [Google Scholar] [CrossRef]
- Yang, H.L.; Chang, W.H.; Chia, Y.C.; Huang, C.J.; Lu, F.J.; Hsu, H.K.; Hseu, Y.C. Toona sinensis extracts induces apoptosis via reactive oxygen species in human promyelocytic leukemia cells. Food Chem. Toxicol. 2006, 44, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.; Barlow, P.J. Quantification of gallic acid and ellagic acid from longan (Dimocarpus longan Lour.) seed and mango (Mangifera indica L.) kernel and their effects on antioxidant activity. Food Chem. 2006, 97, 524–530. [Google Scholar] [CrossRef]
- Oliveira, M.S.C.; Alexandrino, C.D.; Queiroz, V.A.; Vieira, M.G.S.; Morais, S.M.; Batista, W.P. Perfil Fitoquímico e Potencial Antioxidante do Pendúnculo de Caju (Anacardium occidentale L.) de Quatros Clones Diferentes. In Proceedings of the XLVIII Congresso Brasileiro de Química, Rio de Janeiro, Brazil, 29 September–3 October 2008; Sociedade Brasileira de Química: Rio de Janeiro, RI, Brasil, 2008. [Google Scholar]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Lis, E.S.; Fedel-Miyasato, C.A.L.; Kassuya, S.A.; Auharek, A.S.N.; Formagiof, C.A.L.; Cardoso, M.O.; Mauro, A.L.; Cunha-Laura, A.C.D.; Monreal, M.C.; Vieira, R.J. Evaluation of anti-inflammatory, immunomodulatory, chemopreventive and wound healing potentials from Schinus terebinthifolius methanolic extract. Rev. Bras. Farmacogn. 2014, 24, 565–575. [Google Scholar]
- Santana, J.S.; Sartorelli, P.; Lago, J.H.G. Isolamento e avaliação do potencial citotóxico de derivados fenólicos de Schinus terebinthifolius Raddi (Anacardiaceae). Quim. Nova 2012, 35, 2245–2248. [Google Scholar] [CrossRef]
- Carvalho, M.G.; Melo, A.G.N.; Aragão, C.F.S.; Raffin, F.N.; Moura, T.F.A.L. Schinus terebinthifolius Raddi: Chemical composition, biological properties and toxicity. Rev. Bras. Plantas Med. 2013, 15, 158–169. [Google Scholar] [CrossRef]
- Bernardes, N.R.; Heggdorne-Araújo, M.; Borges, I.F.J.C.; Almeida, F.M.; Amaral, E.P.; Lasunskaia, E.B.; Muzitano, M.F.; Oliveira, D.B. Nitric oxide production, inhibitory, antioxidant and antimycobacterial activities of the fruits extract and flavonoid content of Schinus terebinthifolius. Rev. Bras. Farmacogn. 2014, 24, 644–650. [Google Scholar] [CrossRef]
- Queiroz, C.R.A.A.; Morais, S.A.L.; Nascimento, E.A. Caracterização dos taninos da aroeira-preta (Myracrodruon urundeuva). Arvore 2002, 26, 485–492. [Google Scholar] [CrossRef]
- Albuquerque, R.J.M.; Leal, L.K.A.M.; Bandeira, M.A.; Viana, G.S.B.; Rodrigue, L.V. Chalcones from Myracrodruon urundeuva are efficacious in guinea pig ovalbumininduced allergic conjunctiviti. Rev. Bras. Farmacogn. 2011, 21, 953–962. [Google Scholar] [CrossRef]
- Zocoler, A.M.D.; Sanches, A.C.C.; Albrecht, I.; Mello, J.C.P. Antioxidant capacity of extracts and isolated compounds from Stryphnodendron obovatum Benth. Braz. J. Pharm. Sci. 2009, 45, 443–452. [Google Scholar] [CrossRef]
- Araujo-Neto, V.; Bomfim, R.R.; Oliveira, V.O.B.; Passos, A.M.P.R.; Oliveira, J.P.R.; Lima, C.A.; Mendes, S.S.; Estevam, C.S.; Thomazzi, S.M. Therapeutic benefits of Sideroxylon obtusifolium (Humb. ex Roem. & Schult.) T.D. Penn., Sapotaceae, in experimental models of pain and inflammation. Rev. Bras. Farmacogn. 2010, 20, 933–938. [Google Scholar]
- Leite, N.S.; Lima, A.P.; Araújo-Neto, V.; Estevam, C.S.; Pantaleão, S.M.; Camargo, E.A.; Fernandes, R.P.M.; Costa, S.K.P.; Muscará, M.N.; Thomazzi, S.M. Avaliação das atividades cicatrizante, anti-inflamatória tópica e antioxidante do extrato etanólico da Sideroxylon obtusifolium (quixabeira). Rev. Bras. Plantas Med. 2015, 17, 164–170. [Google Scholar] [CrossRef]
- Haslam, E. Chemistry of Vegetable Tanins; Academic Press: London, UK, 1966; p. 68. [Google Scholar]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. PAIN 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Montanher, A.B.; Zucolotto, S.M.; Schenkel, E.P.; Frode, T.S. Evidence of anti-inflammatory effects of Passifloraedulis in an inflammation model. J. Ethnopharmacol. 2007, 109, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Pagano, R.L.; Dias, M.A.A.; Dale, C.S.; Giorgi, R. Neutrophils and the calcium-binding protein MRP-14 mediate carrageenan-induced antinociception in mice. Mediat. Inflammat. 2002, 11, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors José Maria Barbosa-Filho and Túlio Flávio Accioly de Lima Moura.









| Run Number | Gradient Elution | |
|---|---|---|
| Time Interval | Proportion of Phase B | |
| 1 | 0–60 min | 5%–100% |
| 2 | 0–60 min | 5%–50% |
| 3 | 0–60 min | 5%–30% |
| 4 | 0–25 min 25.01–60 min | 5%–15% 15%–20% |
| 5 | 0–14 min 14.01–30 min 30.01–60 min | 7%–12% 12%–12% 12%–25% |
| 6 | 0–10 min 10.01–30 min | 7%–11% 11%–11% |
| 7 * | 0–18 min 18.01–35 min 36.00–45 min | 7%–12% 12%–12% 7%–7% |
| Result of Analysis with EtOAc Phase of A. occidentale | Result of Coinjection Analysis | |||
|---|---|---|---|---|
| Retention Time (min) | Area (mAU.s) | Coinjection with | Retention Time (min) | Area (mAU.s) |
| 6.79 | 19,837.2 | Gallic acid | 6.72 | 26,015.2 |
| 19.83 | 167.1 | Catechin | 19.48 | 540.8 |
| 28.44 | 1447 | Epicatechin | 28.36 | 3293 |
| 30.96 | 2918 | Epigallocatechin | 31.8 | 11,233.5 |
| Compound | Concentration Level (μg/mL) | Expected Concentration (μg/mL) | Recovered Concentration (μg/mL) | Recovery Percentage (%) | RSD * (%) |
|---|---|---|---|---|---|
| 100 | 482.90 | 426.22 | 88.26 | 1.27 | |
| Gallic acid | 200 | 582.90 | 501.78 | 86.10 | 2.98 |
| 300 | 682.91 | 492.59 | 72.13 | 1.74 | |
| 10 | 22.80 | 21.47 | 94.17 | 1.85 | |
| Catechin | 20 | 32.79 | 32.51 | 99.13 | 0.99 |
| 40 | 52.80 | 50.58 | 95.79 | 4.94 | |
| 25 | 46.19 | 47.14 | 102.06 | 1.35 | |
| Epicatechin | 50 | 71.19 | 71.86 | 100.95 | 2.02 |
| 75 | 96.19 | 96.02 | 99.82 | 4.52 |
| Suggestive Peak | Equation of Line | Peak Area (mAU.s) | Quantitation (μg/mL) | Concentration in Extract (μg/g) |
|---|---|---|---|---|
| Gallic acid | y = 57,270x + 565,300 (R2 = 0.9968; RSD = 3%) | 23,336,667 | 397.61 | 198.81 |
| Catechin | y = 16,770x − 8922 (R2 = 0.9960; RSD = 4.9%) | 222,170.2 | 13.78 | 6.89 |
| Epicatechin | y = 16,780x − 22,620 (R2 = 0.9935; RSD = 5%) | 367,368.8 | 23.24 | 11.62 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilar, M.S.d.A.; De Souza, G.L.; Vilar, D.D.A.; Leite, J.A.; Raffin, F.N.; Barbosa-Filho, J.M.; Nogueira, F.H.A.; Rodrigues-Mascarenhas, S.; Moura, T.F.A.d.L. Assessment of Phenolic Compounds and Anti-Inflammatory Activity of Ethyl Acetate Phase of Anacardium occidentale L. Bark. Molecules 2016, 21, 1087. https://doi.org/10.3390/molecules21081087
Vilar MSdA, De Souza GL, Vilar DDA, Leite JA, Raffin FN, Barbosa-Filho JM, Nogueira FHA, Rodrigues-Mascarenhas S, Moura TFAdL. Assessment of Phenolic Compounds and Anti-Inflammatory Activity of Ethyl Acetate Phase of Anacardium occidentale L. Bark. Molecules. 2016; 21(8):1087. https://doi.org/10.3390/molecules21081087
Chicago/Turabian StyleVilar, Marina Suênia de Araújo, Graziene Lopes De Souza, Daniela De Araújo Vilar, Jacqueline Alves Leite, Fernanda Nervo Raffin, José Maria Barbosa-Filho, Fernando Henrique Andrade Nogueira, Sandra Rodrigues-Mascarenhas, and Túlio Flávio Accioly de Lima Moura. 2016. "Assessment of Phenolic Compounds and Anti-Inflammatory Activity of Ethyl Acetate Phase of Anacardium occidentale L. Bark" Molecules 21, no. 8: 1087. https://doi.org/10.3390/molecules21081087
APA StyleVilar, M. S. d. A., De Souza, G. L., Vilar, D. D. A., Leite, J. A., Raffin, F. N., Barbosa-Filho, J. M., Nogueira, F. H. A., Rodrigues-Mascarenhas, S., & Moura, T. F. A. d. L. (2016). Assessment of Phenolic Compounds and Anti-Inflammatory Activity of Ethyl Acetate Phase of Anacardium occidentale L. Bark. Molecules, 21(8), 1087. https://doi.org/10.3390/molecules21081087