Red Grape Skin Polyphenols Blunt Matrix Metalloproteinase-2 and -9 Activity and Expression in Cell Models of Vascular Inflammation: Protective Role in Degenerative and Inflammatory Diseases
Abstract
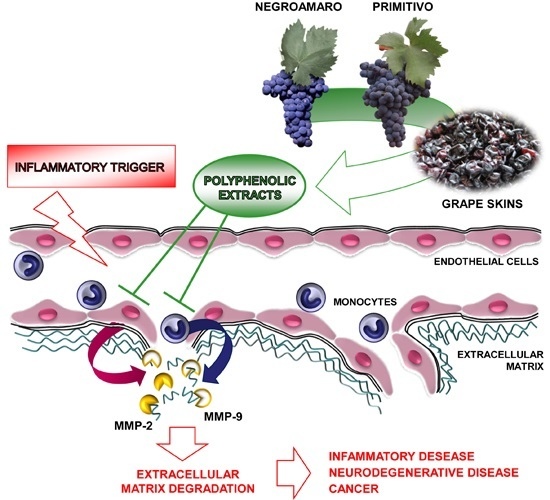
:1. Introduction
2. Results
2.1. Polyphenolic Profile and Antioxidant Property of Negroamaro and Primitivo Grape Skin Extracts
2.2. Red Grape Skin Polyphenol Extracts Prevent MMP-9 and MMP-2 Gelatinolytic Activity and Cell Invasion in Inflamed Endothelium
2.3. Red Grape Skin Polyphenol Extracts Suppress MMP-9 Release and Expression in Inflammatory Monocytes
2.4. Specific Red Grape Skin Polyphenols Differently Modulate Gelatinase Activity and Expression in Inflamed Endothelial Cells and Monocytes
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Preparation of Polyphenolic Extracts
4.3. Identification and Quantification of Phenolic Compounds
4.4. Antioxidant Activity of Grape Skin Polyphenolic Extracts
4.5. Cell Culture and Treatment
4.6. Gelatinase Activity
4.7. MMP-9 and MMP-2 Protein Release
4.8. Cell Invasion Assay
4.9. Quantitative Reverse Transcription-Polymerase Chain Reaction Analysis
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Merdad, A.; Karim, S.; Schulten, H.J.; Dallol, A.; Buhmeida, A.; Al-Thubaity, F.; Gari, M.A.; Chaudhary, A.G.; Abuzenadah, A.M.; Al-Qahtani, M.H. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer: MMP-9 as a potential biomarker for cancer invasion and metastasis. Anticancer Res. 2014, 34, 1355–1366. [Google Scholar] [PubMed]
- Newby, A.C. Metalloproteinases promote plaque rupture and myocardial infarction: A persuasive concept waiting for clinical translation. Matrix Biol. 2015, 44–46, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009, 8, 205–216. [Google Scholar] [CrossRef]
- Nissinen, L.; Kahari, V.M. Matrix metalloproteinases in inflammation. Biochim. Biophys Acta 2014, 1840, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.P.; Hwang, J.J.; Murphy, G.; Ip, M.M. Functional significance of MMP-9 in tumor necrosis factor-induced proliferation and branching morphogenesis of mammary epithelial cells. Endocrinology 2000, 141, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Waas, E.T.; Lomme, R.M.; DeGroot, J.; Wobbes, T.; Hendriks, T. Tissue levels of active matrix metalloproteinase-2 and -9 in colorectal cancer. Br. J. Cancer 2002, 86, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Wagsater, D.; Zhu, C.; Bjorkegren, J.; Skogsberg, J.; Eriksson, P. MMP-2 and MMP-9 are prominent matrix metalloproteinases during atherosclerosis development in the Ldlr−/−Apob100/100 mouse. Int. J. Mol. Med. 2011, 28, 247–253. [Google Scholar] [PubMed]
- Loftus, I.M.; Naylor, A.R.; Goodall, S.; Crowther, M.; Jones, L.; Bell, P.R.; Thompson, M.M. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke 2000, 31, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [PubMed]
- Ogita, M.; Miyauchi, K.; Morimoto, T.; Daida, H.; Kimura, T.; Hiro, T.; Nakagawa, Y.; Yamagishi, M.; Ozaki, Y.; Matsuzaki, M. Association between circulating matrix metalloproteinase levels and coronary plaque regression after acute coronary syndrome—Subanalysis of the JAPAN-ACS study. Atherosclerosis 2013, 226, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lorenzl, S.; Albers, D.S.; Relkin, N.; Ngyuen, T.; Hilgenberg, S.L.; Chirichigno, J.; Cudkowicz, M.E.; Beal, M.F. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem. Int. 2003, 43, 191–196. [Google Scholar] [CrossRef]
- Hernandez-Guillamon, M.; Mawhirt, S.; Blais, S.; Montaner, J.; Neubert, T.A.; Rostagno, A.; Ghiso, J. Sequential Amyloid-beta Degradation by the Matrix Metalloproteases MMP-2 and MMP-9. J. Biol. Chem. 2015, 290, 15078–15091. [Google Scholar] [CrossRef] [PubMed]
- Newby, A.C. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb. Vasc. Biol. 2008, 28, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Perlstein, T.S.; Lee, R.T. Smoking, metalloproteinases, and vascular disease. Arterioscler Thromb. Vasc. Biol. 2006, 26, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Asanuma, K.; Godin, D.; Meng, X. N-Acetyl-cysteine decreases the matrix-degrading capacity of macrophage-derived foam cells: New target for antioxidant therapy? Circulation 1998, 97, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Brossard, M.; Page, M.; Gingras, D.; Beliveau, R. Matrix metalloproteinase inhibition by green tea catechins. Biochim. Biophys. Acta 2000, 1478, 51–60. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Canavesi, M.; Galli, G.; Bellosta, S. Dietary polyphenols and regulation of gelatinase expression and activity. Thromb Haemost 2005, 93, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Zhang, Y.; Tonelli, C.; Petroni, K. Plants, diet, and health. Annu. Rev. Plant Biol. 2013, 64, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Szmitko, P.E.; Verma, S. Antiatherogenic potential of red wine: clinician update. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Etienne-Selloum, N.; Sarr, M.; Kane, M.O.; Beretz, A.; Schini-Kerth, V.B. Angiotensin II induces the vascular expression of VEGF and MMP-2 in vivo: Preventive effect of red wine polyphenols. J. Vasc. Res. 2008, 45, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, G.; Grieco, F. Functional Properties of Grape and Wine Polyphenols. Plant Food. Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; El Bedoui, J.; Anglard, P.; Schini-Kerth, V.B. Red wine polyphenolic compounds strongly inhibit pro-matrix metalloproteinase-2 expression and its activation in response to thrombin via direct inhibition of membrane type 1-matrix metalloproteinase in vascular smooth muscle cells. Circulation 2004, 110, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; El Bedoui, J.; Schini-Kerth, V.B. Antiangiogenic properties of natural polyphenols from red wine and green tea. J. Nutr. Biochem. 2005, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Pellegrino, M.; Storelli, C.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Multiple anti-inflammatory and anti-atherosclerotic properties of red wine polyphenolic extracts: Differential role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression. Eur. J. Nutr. 2016, 55, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; de Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Rocha, K.K.; Souza, G.A.; Seiva, F.R.; Ebaid, G.X.; Novelli, E.L. Weekend ethanol consumption and high-sucrose diet: Resveratrol effects on energy expenditure, substrate oxidation, lipid profile, oxidative stress and hepatic energy metabolism. Alcohol Alcohol. 2011, 46, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; de Caterina, R. Alcohol and atherosclerosis: A double edged sword. Vascul. Pharmacol. 2012, 57, 65–68. [Google Scholar] [CrossRef] [PubMed]
- De Nisco, M.; Manfra, M.; Bolognese, A.; Sofo, A.; Scopa, A.; Tenore, G.C.; Pagano, F.; Milite, C.; Russo, M.T. Nutraceutical properties and polyphenolic profile of berry skin and wine of Vitis vinifera L. (cv. Aglianico). Food Chem. 2013, 140, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Khymenets, O.; Andres-Lacueva, C.; Urpi-Sarda, M.; Vazquez-Fresno, R.; Mart, M.M.; Reglero, G.; Torres, M.; Llorach, R. Metabolic fingerprint after acute and under sustained consumption of a functional beverage based on grape skin extract in healthy human subjects. Food Funct. 2015, 6, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Bartolome, B.; Gomez-Cordoves, C. Updated knowledge about the presence of phenolic compounds in wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118. [Google Scholar] [CrossRef] [PubMed]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine by-products: Phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different French grape varieties. Molecules 2013, 19, 482–506. [Google Scholar] [CrossRef] [PubMed]
- Vitseva, O.; Varghese, S.; Chakrabarti, S.; Folts, J.D.; Freedman, J.E. Grape seed and skin extracts inhibit platelet function and release of reactive oxygen intermediates. J. Cardiovasc. Pharmacol. 2005, 46, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Barthomeuf, C.; Lamy, S.; Blanchette, M.; Boivin, D.; Gingras, D.; Beliveau, R. Inhibition of sphingosine-1-phosphate- and vascular endothelial growth factor-induced endothelial cell chemotaxis by red grape skin polyphenols correlates with a decrease in early platelet-activating factor synthesis. Free Radic. Biol. Med. 2006, 40, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; di Colo, G.; D’Onofrio, C.; Fausto, C.; Balbarini, A.; di Stefano, R. Red grape skin and seeds polyphenols: Evidence of their protective effects on endothelial progenitor cells and improvement of their intestinal absorption. Eur. J. Pharm. Biopharm. 2012, 80, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Houseman, E.J.; George, T.W.; Corona, G.; Garnotel, R.; Jackson, K.G.; Sellier, C.; Gillery, P.; Kennedy, O.B.; Lovegrove, J.A.; et al. Moderate Champagne consumption promotes an acute improvement in acute endothelial-independent vascular function in healthy human volunteers. Br. J. Nutr. 2010, 103, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, L.; Shi, Y.; Sun, A.; Xu, F.; Chi, J.; Huang, D. Chinese yellow wine and red wine inhibit matrix metalloproteinase-2 and improve atherosclerotic plaque in LDL receptor knockout mice. Cardiovasc. Ther. 2010, 28, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Etienne-Selloum, N.; Brasse, D.; Khallouf, H.; Bronner, C.; Rio, M.C.; Beretz, A.; Schini-Kerth, V.B. Intake of grape-derived polyphenols reduces C26 tumor growth by inhibiting angiogenesis and inducing apoptosis. FASEB J. 2010, 24, 3360–3369. [Google Scholar] [CrossRef] [PubMed]
- Boussenna, A.; Cholet, J.; Goncalves-Mendes, N.; Joubert-Zakeyh, J.; Fraisse, D.; Vasson, M.P.; Texier, O.; Felgines, C. Polyphenol-rich grape pomace extracts protect against dextran sulfate sodium-induced colitis in rats. J. Sci. Food Agric. 2016, 96, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008, 75, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Seiki, M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene 1993, 8, 395–405. [Google Scholar] [PubMed]
- Takada, Y.; Singh, S.; Aggarwal, B.B. Identification of a p65 peptide that selectively inhibits NF-κB activation induced by various inflammatory stimuli and its role in down-regulation of NF-κB-mediated gene expression and up-regulation of apoptosis. J. Biol. Chem. 2004, 279, 15096–15104. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.; Fabunmi, R.P.; Baker, A.H.; Newby, A.C. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-κB. FEBS Lett. 1998, 435, 29–34. [Google Scholar] [CrossRef]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; de Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Shen, F.; Liu, B.H.; Cheng, G.F. Resveratrol inhibits matrix metalloproteinase-9 transcription in U937 cells. Acta Pharmacol. Sin. 2003, 24, 1167–1171. [Google Scholar] [PubMed]
- Pilatova, M.; Stupakova, V.; Varinska, L.; Sarissky, M.; Mirossay, L.; Mirossay, A.; Gal, P.; Kraus, V.; Dianiskova, K.; Mojzis, J. Effect of selected flavones on cancer and endothelial cells. Gen. Physiol. Biophys. 2010, 29, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Lee, E.G.; Lee, H.; Cho, I.J.; Choi, Y.J.; Sung, M.S.; Yoo, H.G.; Yoo, W.H. Kaempferol inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int. J. Mol. Med. 2013, 32, 971–977. [Google Scholar] [PubMed]
- Ko, S.Y. Myricetin suppresses LPS-induced MMP expression in human gingival fibroblasts and inhibits osteoclastogenesis by downregulating NFATc1 in RANKL-induced RAW 264.7 cells. Arch. Oral Biol. 2012, 57, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Lim, J.H.; Kim, Y.H.; Suh, S.I.; Min, D.S.; Chang, J.S.; Lee, Y.H.; Park, J.W.; Kwon, T.K. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene 2004, 23, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zheng, X.Y.; Ye, J.; Wu, T.T.; Wang, J.; Chen, W. Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutr. Cancer 2012, 64, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Remesy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Shimoi, K.; Saka, N.; Nozawa, R.; Sato, M.; Amano, I.; Nakayama, T.; Kinae, N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab. Dispos. 2001, 29, 1521–1524. [Google Scholar] [PubMed]
- Yoo, Y.J.; Saliba, A.J.; Prenzler, P.D. Should Red Wine Be Considered a Functional Food? Compr. Rev. Food Sci. Food Saf. 2010, 9, 530–551. [Google Scholar] [CrossRef]
- Vitaglione, P.; Sforza, S.; Galaverna, G.; Ghidini, C.; Caporaso, N.; Vescovi, P.P.; Fogliano, V.; Marchelli, R. Bioavailability of trans-resveratrol from red wine in humans. Mol. Nutr. Food Res. 2005, 49, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Kraft, T.E.; Parisotto, D.; Schempp, C.; Efferth, T. Fighting cancer with red wine? Molecular mechanisms of resveratrol. Crit. Rev. Food Sci. Nutr. 2009, 49, 782–799. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [PubMed]
- Fournand, D.; Vicens, A.; Sidhoum, L.; Souquet, J.M.; Moutounet, M.; Cheynier, V. Accumulation and extractability of grape skin tannins and anthocyanins at different advanced physiological stages. J. Agric. Food Chem. 2006, 54, 7331–7338. [Google Scholar] [CrossRef] [PubMed]
- D’introno, A.; Paradiso, A.; Scoditti, E.; D’Amico, L.; de Paolis, A.; Carluccio, M.A.; Nicoletti, I.; DeGara, L.; Santino, A.; Giovinazzo, G. Antioxidant and anti-inflammatory properties of tomato fruits synthesizing different amounts of stilbenes. Plant Biotechnol. J. 2009, 7, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Ancora, M.A.; Massaro, M.; Carluccio, M.; Scoditti, E.; Distante, A.; Storelli, C.; de Caterina, R. Homocysteine induces VCAM-1 gene expression through NF-κB and NAD(P)H oxidase activation: Protective role of Mediterranean diet polyphenolic antioxidants. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2344–H2354. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.




| Polyphenols | NSPE | PSPE | ||
|---|---|---|---|---|
| µg/g Dry Weight | (%) | µg/g Dry Weight | (%) | |
| Flavonols | 21.0 | 17.5 * | ||
| Kaempferol | 25.9 ± 0.4 | 0.3 | 209.7 ± 1.1 | 2.5 * |
| Kaempferol-3-O-glucoside | 209.8 ± 1.5 | 2.9 | 180.2 ± 1.3 | 2.2 |
| Quercetin | 150.2 ± 1.5 | 2.1 | 118.8 ± 1.2 | 1.4 * |
| Quercetin-3-O-glucoside | 908.8 ± 5.5 | 12.5 | 650.5 ± 4.1 | 7.8 * |
| Quercetin-3-O-rutinoside | 180.8 ± 1.4 | 2.5 | 248.7 ± 1.5 | 3.0 * |
| Myricetin-3-O-glucoside | 48.8 ± 0.5 | 0.7 | 54.1 ± 0.4 | 0.6 |
| Stilbenes | 7.8 | 5.2 * | ||
| Trans-Resveratrol | 30.6 ± 0.4 | 0.4 | 54.3 ± 0.9 | 0.6 * |
| Trans-Piceid | 536.8 ± 2.2 | 7.4 | 378.2 ± 2.5 | 4.5 * |
| Phenolic acids | 71.2 | 77.3 | ||
| p-Coumaric acid | 2488.8 ± 5.5 | 32.2 | 2989.8 ± 5.5 | 35.9 |
| Caftaric acid | 1200.2 ± 2.9 | 16.5 | 998.8 ± 7.8 | 12.0 |
| Gallic acid | 1487.9 ± 2.8 | 20.5 | 2447.8 ± 3.5 | 29.4 * |
| Total | 7268.6 | 8327.4 | ||
| LAA | HAA | Total | |
|---|---|---|---|
| µmoles TE/g Dry Weight | |||
| NSPE | 1.80 ± 0.01 | 17.65 ± 0.23 | 19.45 |
| PSPE | 6.32 ± 0.04 * | 16.47 ± 0.52 | 22.79 |
| NSPE (µg/mL) | PSPE (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 5 | 25 | 5 | 25 | ||||
| STRUCTURE | POLYPHENOLS | R1 | R2 | µmol/L | |||
| FLAVONOLS | |||||||
 | Kaempferol (KMP) | H | H | 0.05 | 0.26 | 0.44 | 2.18 |
| Quercetin (QRC) | OH | H | 0.35 | 1.74 | 0.23 | 1.16 | |
| STILBENES | |||||||
 | trans-Resveratrol (RSV) | OH | - | 0.09 | 0.44 | 0.13 | 0.66 |
| trans-Piceid (PCD) | OGlucose | - | 0.95 | 4.74 | 0.58 | 2.88 | |
| SOLUBLE ACIDS | |||||||
 | p-Coumaric acid (CMR) | H | OH | 9.81 | 49.04 | 10.93 | 54.67 |
| Caftaric acid (CFT) | OH | C4O6H5 | 2.64 | 13.22 | 1.92 | 9.61 | |
| Gene Name | Accession Number | Forward Primer | Reverse Primer | Size (bp) |
|---|---|---|---|---|
| MMP-9 | NM_004994.2 | 5′-AAAGCCTATTTCTGCCAGGAC-3′ | 5′-GTGGGGATTTACATGGCACT-3′ | 157 |
| MMP-2 | NM_004530.4 | 5′-CACTTTCCTGGGCAACAAAT-3′ | 5′-TGATGTCATCCTGGGACAGA-3′ | 257 |
| TIMP-1 | NM_003254.2 | 5′-TGACATCCGGTTCGTCTACA-3′ | 5′-CTGCAGTTTTCCAGCAATGA-3′ | 103 |
| TIMP-2 | NM_003255.4 | 5′-CCAAGCAGGAGTTTCTCGAC-3′ | 5′-TTTCCAGGAAGGGATGTCAG-3′ | 121 |
| GAPDH | NM_002046.3 | 5′-ATCACTGCCACCCAGAAGAC-3′ | 5′-TTCTAGACGGCAGGTCAGGT-3′ | 210 |
| 18 rRNA | NR_003286.2 | 5′-AAACGGCTACCACATCCAAG-3′ | 5′-CCTCCAATGGATCCTCGTTA-3′ | 155 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabriso, N.; Massaro, M.; Scoditti, E.; Pellegrino, M.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Red Grape Skin Polyphenols Blunt Matrix Metalloproteinase-2 and -9 Activity and Expression in Cell Models of Vascular Inflammation: Protective Role in Degenerative and Inflammatory Diseases. Molecules 2016, 21, 1147. https://doi.org/10.3390/molecules21091147
Calabriso N, Massaro M, Scoditti E, Pellegrino M, Ingrosso I, Giovinazzo G, Carluccio MA. Red Grape Skin Polyphenols Blunt Matrix Metalloproteinase-2 and -9 Activity and Expression in Cell Models of Vascular Inflammation: Protective Role in Degenerative and Inflammatory Diseases. Molecules. 2016; 21(9):1147. https://doi.org/10.3390/molecules21091147
Chicago/Turabian StyleCalabriso, Nadia, Marika Massaro, Egeria Scoditti, Mariangela Pellegrino, Ilaria Ingrosso, Giovanna Giovinazzo, and Maria Annunziata Carluccio. 2016. "Red Grape Skin Polyphenols Blunt Matrix Metalloproteinase-2 and -9 Activity and Expression in Cell Models of Vascular Inflammation: Protective Role in Degenerative and Inflammatory Diseases" Molecules 21, no. 9: 1147. https://doi.org/10.3390/molecules21091147
APA StyleCalabriso, N., Massaro, M., Scoditti, E., Pellegrino, M., Ingrosso, I., Giovinazzo, G., & Carluccio, M. A. (2016). Red Grape Skin Polyphenols Blunt Matrix Metalloproteinase-2 and -9 Activity and Expression in Cell Models of Vascular Inflammation: Protective Role in Degenerative and Inflammatory Diseases. Molecules, 21(9), 1147. https://doi.org/10.3390/molecules21091147