Formation of Hydrogen Sulfide in Wine: Interactions between Copper and Sulfur Dioxide
Abstract
:1. Introduction
2. Results
2.1. Dissolved Oxygen
2.2. Formation of Hydrogen Sulfide in Wine
2.2.1. The Effect of Cu2+ on H2S Formation
2.2.2. The Effect of SO2 on H2S Formation
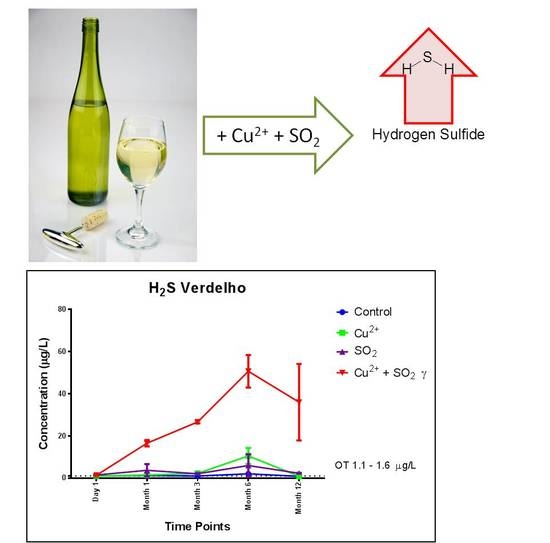
2.2.3. The Effects of Cu2+ and SO2 Interaction on H2S Formation
2.3. Interaction of Cu2+ and SO2 in Model Wine
2.3.1. Formation of H2S in Model Wine
2.3.2. Reactions between H2S and SO2 with a Model Wine Compound/Thiol Quenching Compound
Competition Reactions between H2S and SO2 with a Model Wine Quinone
Competition Reactions between a Model Wine Quinone with H2S and SO2 at Wine Relevant Concentrations
3. Discussion
3.1. Impact of Oxygen on the Formation of H2S from Cu2+ + SO2 Interaction in This Study
3.2. Formation of H2S Associated with Cu2+ + SO2 Treatment
3.3. Mechanisms Modulating H2S Formation
4. Materials and Methods
4.1. Materials
4.2. Wine Samples
4.3. Chemical Analyses
4.3.1. Oxygen Measurement
4.3.2. Metal Quantifications
4.3.3. Determination of SO2
4.3.4. Preparation of Reaction Products of H2S and SO2 with 4-Methylbenzoquinone
4.3.5. Analysis of 4-Methylbenzoquinone Adducts Using Liquid Chromatography
4.3.6. Gas Chromatography Coupled to Sulfur Chemiluminescence Detection
4.4. Sample Preparation and Analysis
Preparation of Verdelho, Shiraz, and Model Wine Samples Spiked with Cu2+ + SO2
4.5. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 4MBQ | 4-methylbenzoquinone |
| 4MC | 4-Methylcatechol |
| ANOVA | Analysis of variance |
| AVG | Average |
| AWRI | Australian Wine Research Institute |
| DO | Dissolved oxygen |
| GSH | Glutathione |
| H2O2 | Hydrogen peroxide |
| H2S | Hydrogen sulfide |
| HPLC | High pressure liquid chromatography |
| LCMS | Liquid chromatography-mass spectrometry |
| LC-HRMS | Liquid chromatography-high resolution mass spectrometry |
| MeSH | Methanethiol |
| MeOH | Methanol |
| OT | Odor threshold |
| PMS | Potassium hydrogen tartrate |
| SO2 | Sulfur dioxide |
| SD | Standard deviation |
| THF | Tetrahydrofuran |
| VSCs | Volatile sulfur compounds |
References
- Smith, M.E.; Bekker, M.Z.; Smith, P.A.; Wilkes, E.N. Sources of volatile sulfur compounds in wine. Aust. J. Grape Wine Res. 2015, 21, 705–712. [Google Scholar] [CrossRef]
- Siebert, T.E.; Bramley, B.; Solomon, M.R. Hydrogen sulfide: Aroma detection threshold study in white and red wine. AWRI Tech. Rev. 2009, 183, 14–16. [Google Scholar]
- Villamor, R.R.; Ross, C.F. Wine matrix compounds affect perception of wine aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M.; Kwiatkowski, M.; Vidal, S.; Capone, D.; Siebert, T.; Dieval, J.B.; Aagaard, O.; Waters, E.J. Evolution of 3-mercaptohexanol, hydrogen sulfide, and methyl mercaptan during bottle storage of Sauvignon blanc wines. Effect of glutathione, copper, oxygen exposure, and closure-derived oxygen. J. Agric. Food Chem. 2011, 59, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Bekker, M.Z.; Day, M.P.; Holt, H.; Wilkes, E.; Smith, P.A. Effect of oxygen exposure during fermentation on volatile sulfur compounds in Shiraz wine and a comparison of strategies for remediation of reductive character. Aust. J. Grape Wine Res. 2016, 22, 24–35. [Google Scholar] [CrossRef]
- Day, M.P.; Schmidt, S.A.; Smith, P.A.; Wilkes, E.N. Use and impact of oxygen during winemaking. Aust. J. Grape Wine Res. 2015, 21, 693–704. [Google Scholar] [CrossRef]
- Franco-Luesma, E.; Ferreira, V. Reductive off-odors in wines: Formation and release of H2S and methanethiol during the accelerated anoxic storage of wines. Food Chem. 2016, 199, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Franco-Luesma, E.; Ferreira, V. Formation and release of H2S, methanethiol, and dimethylsulfide during the anoxic storage of wines at room temperature. J. Agric. Food Chem. 2016, 64, 6317–6326. [Google Scholar] [CrossRef] [PubMed]
- Nedjma, M.; Hoffmann, N. Hydrogen sulfide reactivity with thiols in the presence of copper(II) in hydroalcoholic solutions cognac brandies: Formation of symmetrical and unsymmetrical dialkyl trisulfides. J. Agric. Food Chem. 1996, 44, 3935–3938. [Google Scholar] [CrossRef]
- Viviers, M.Z.; Smith, M.E.; Wilkes, E.; Smith, P. Effects of five metals on the evolution of hydrogen sulfide, methanethiol, and dimethyl sulfide during anaerobic storage of Chardonnay and Shiraz wines. J. Agric. Food Chem. 2013, 61, 12385–12396. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D. The influence of metal-ions on concentrations of flavor-active sulfur-compounds measured in beer using dynamic headspace sampling. J. Sci. Food Agric. 1995, 67, 25–28. [Google Scholar] [CrossRef]
- Kreitman, G.Y.; Danilewicz, J.C.; Jeffery, D.W.; Elias, R.J. Reaction mechanisms of metals with hydrogen sulfide and thiols in model wine. Part 1: Copper-catalyzed oxidation. J. Agric. Food Chem. 2016, 64, 4095–4104. [Google Scholar] [PubMed]
- Clark, A.C.; Grant-Preece, P.; Cleghorn, N.; Scollary, G.R. Copper(II) addition to white wines containing hydrogen sulfide: Residual copper concentration and activity. Aust. J. Grape Wine Res. 2015, 21, 30–39. [Google Scholar] [CrossRef]
- Clark, A.C.; Kontoudakis, N.; Barril, C.; Schmidtke, L.M.; Scollary, G.R. Measurement of labile copper in wine by medium exchange stripping potentiometry utilising screen printed carbon electrodes. Talanta 2016, 154, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Bekker, M.Z.; Mierczynska-Vasilev, A.; Smith, P.A.; Wilkes, E.N. The effects of pH and copper on the formation of volatile sulfur compounds in Chardonnay and Shiraz wines post-bottling. Food Chem. 2016, 207, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine Science Principles and Applications, 3rd ed.; Elsevier Inc.: San Diego, CA, USA, 2008; p. 307. [Google Scholar]
- Danilewicz, J.C. Mechanism of autoxidation of polyphenols and participation of sulfite in wine: Key role of iron. Am. J. Enol. Vitic. 2011, 62, 319–328. [Google Scholar] [CrossRef]
- Elias, R.J.; Waterhouse, A.L. Controlling the fenton reaction in wine. J. Agric. Food Chem. 2010, 58, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Danilewicz, J.C. Reactions involving iron in mediating catechol oxidation in model wine. Am. J. Enol. Vitic. 2013, 64, 316–324. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of wine phenolics: A critical evaluation and hypotheses. Am. J. Enol. Vitic. 2006, 53, 306–313. [Google Scholar]
- Nikolantonaki, M.; Waterhouse, A.L. A method to quantify quinone reaction rates with wine relevant nucleophiles: A key to the understanding of oxidative loss of varietal thiols. J. Agric. Food Chem. 2012, 60, 8484–8491. [Google Scholar] [CrossRef] [PubMed]
- Nikolantonaki, M.; Magiatis, P.; Waterhouse, A.L. Measuring protection of aromatic wine thiols from oxidation by competitive reactions vs wine preservatives with ortho-quinones. Food Chem. 2014, 163, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Rankine, B.C. Making Good Wine; Pan Macmillan Australia Pt Limited: Sydney, Australia, 2004; pp. 96–99. [Google Scholar]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbok of Enology: The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume 2, pp. 103, 393. [Google Scholar] [CrossRef]
- Lopes, P.; Silva, M.A.; Pons, A.; Tominaga, T.; Lavigne, V.; Saucier, C.; Darriet, P.; Teissedre, P.L.; Dubourdieu, D. Impact of oxygen dissolved at bottling and transmitted through closures on the composition and sensory properties of a sauvignon blanc wine during bottle storage. J. Agric. Food Chem. 2009, 57, 10261–10270. [Google Scholar] [CrossRef] [PubMed]
- Nikolantonaki, M.; Jourdes, M.; Shinoda, K.; Teissedre, P.L.; Quideau, S.; Darriet, P. Identification of adducts between an odoriferous volatile thiol and oxidized grape phenolic compounds: Kinetic study of adduct formation under chemical and enzymatic oxidation conditions. J. Agric. Food Chem. 2012, 60, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M. Oxygen contribution to wine aroma evolution during bottle aging. J. Agric. Food Chem. 2013, 61, 6125–6136. [Google Scholar] [CrossRef] [PubMed]
- Siebert, T.E.; Solomon, M.R.; Pollnitz, A.P.; Jeffery, D.W. Selective determination of volatile sulfur compounds in wine by gas chromatography with sulfur chemiluminescence detection. J. Agric. Food Chem. 2010, 58, 9454–9462. [Google Scholar] [CrossRef] [PubMed]
- Danilewicz, J.C. Interaction of sulfur dioxide, polyphenols, and oxygen in a wine-model system: Central role of iron and copper. Am. J. Enol. Vitic. 2007, 58, 53–60. [Google Scholar]
- Danilewicz, J.C.; Seccombe, J.T.; Whelan, J. Mechanism of interaction of polyphenols, oxygen, and sulfur dioxide in model wine and wine. Am. J. Enol. Vitic. 2008, 59, 128–136. [Google Scholar]
- Rauhut, D.; Kurbel, H.; Dittrich, H.H. Sulfur compounds and their influence on wine quality. Wein Wiss. 1993, 48, 214–218. [Google Scholar]
- Franco-Luesma, E.; Ferreira, V. Quantitative analysis of free and bonded forms of volatile sulfur compouds in wine. Basic methodologies and evidences showing the existence of reversible cation-complexed forms. J. Chromatogr. A 2014, 1359, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Thiel, G.; Geisler, G.; Blechschmidt, I.; Danzer, K. Determination of trace elements in wines and classification according to their provenance. Anal. Bioanal. Chem. 2004, 378, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.E.; Watling, R.J.; Lee, G.S. The multi-element determination and regional discrimination of Australian wines. Food Chem. 2012, 133, 1081–1089. [Google Scholar] [CrossRef]
- Analytical Requirements for the Export of Australian Wine. The Australian Wine Research Institute, 2016. Available online: http://www.awri.com.au/industry_support/regulatory_assistance/export_requirements/ (accessed on 29 June 2016).
- Peterson, G.F.; Kirrane, M.; Hill, N.; Agapito, A. A comprehensive survey of the total sulfur dioxide concentration of American wines. Am. J. Enol. Vitic. 2000, 51, 189–191. [Google Scholar]
- Jongberg, S.; Gislason, N.E.; Lund, M.N.; Skibsted, L.H.; Waterhouse, A.L. Thiol-quinone adduct formation in myofibrillar proteins detected by lc-ms. J. Agric. Food Chem. 2011, 59, 6900–6905. [Google Scholar] [CrossRef] [PubMed]
- Fisher, U.; Noble, A.C. The effect of ethanol, catechin concetration, and pH on sourness and bitterness of wine. Am. J. Enol. Vitic. 1994, 45, 6–10. [Google Scholar]
- Komes, D.; Ulrich, D.; Kovacevic, G.; Lovric, T. Study of phenolic and volatile composition of white wine during fermentaion and a short time of storage. Vitis 2007, 46, 77–84. [Google Scholar]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics-adaptation and validation of methyl cellulose precipitable tannin assay and modified Somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.





| Verdelho Wine Samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2S (μg/L) | DAY 0 | MONTH 1 | MONTH 3 | MONTH 6 | MONTH 12 | Overall Significance | |||||
| AVG 1 | SD 2 | AVG | SD | AVG | SD | AVG | SD | AVG | SD | ||
| Control | 1.122 | 0.040 | 1.270 | 0.026 | 1.066 | 0.136 | 2.058 | 0.012 | 0.879 | 0.028 | |
| Cu2+ | 0.981 | 0.062 | 1.523 | 0.062 | 2.276 | 0.428 | 10.53 | 3.776 | 0.923 | 0.395 | ns |
| SO2 | 1.538 | 0.042 | 3.764 | 2.868 | 2.139 | 0.235 | 5.991 | 5.312 | 2.318 | 0.222 | ns |
| Cu2+ + SO2 | 1.338 | 0.137 | 16.59 | 1.527 | 26.64 | 0.683 | 50.62 | 7.685 | 36.01 | 18.09 | **** (+) 3 |
| Shiraz Wine Samples | |||||||||||
| H2S (μg/L) | DAY 0 | MONTH 1 | MONTH 3 | MONTH 6 | MONTH 12 | Overall Significance | |||||
| AVG | SD | AVG | SD | AVG | SD | AVG | SD | AVG | SD | ||
| Control | 2.271 | 0.058 | 1.672 | 0.166 | 2.474 | 0.241 | 2.604 | 0.033 | 1.515 | 0.195 | |
| Cu2+ | 2.063 | 0.066 | 4.202 | 0.354 | 14.71 | 1.701 | 28.50 | 1.048 | 12.41 | 11.00 | **** (+) 4 |
| SO2 | 2.254 | 0.053 | 2.900 | 0.615 | 3.162 | 0.032 | 2.676 | 0.244 | 2.095 | 0.576 | ns |
| Cu2+ + SO2 | 2.096 | 0.101 | 18.31 | 0.886 | 4.594 | 0.977 | 25.77 | 2.364 | 27.49 | 10.75 | **** (+) 4 |
| H2S-4MC and SO2-4MC Adducts 1 | Measured HRMS 2 | Predicted HRMS 3 | Prediction Accuracy (err, mDa) 4 | Published HRMS 5 | Peak Ratio 6 |
|---|---|---|---|---|---|
| H2S-4MC adducts: 1mM 4MBQ + 4mM H2S | |||||
| 4MC dimer + SH; C14H13O4S | 277.0543 | 277.054 | −0.3 | 277.0524 | 5 |
| 4MC dimer + SH; C14H13O4S | 277.0542 | 277.054 | −0.2 | 277.0524 | 3 |
| 4MC dimer + 2SH; C14H13O4S2 | 309.0259 | 309.0261 | 0.1 | 309.0240 | 1 |
| SO2-4MC adducts: 1 mM 4MBQ + 4 mM SO2 | |||||
| 4MC + SO3; C7H7O5S | 203.0024 | 203.0020 | −0.4 | 203.0019 | 2 |
| 4MC + SO3; C7H7O5S | 203.0022 | 203.0020 | −0.2 | 203.0019 | 1 |
| 4MC dimer + SO3; C14H13O7S | 325.0389 | 325.0387 | −0.1 | n/a | 3 |
| 4MC dimer + SO3; C14H13O7S | 325.0381 | 325.0387 | 0.7 | n/a | 1 |
| H2S-4MC and SO2-4MC adducts: 1 mM 4MBQ + 2 mM H2S + 2 mM SO2 | |||||
| 4MC dimer + SH; C14H13O4S | 277.0542 | 277.054 | −0.2 | 277.0524 | 4 |
| 4-MC + SO3; C7H7O5S | 203.0022 | 203.0020 | −0.2 | 203.0019 | 1 |
| 4MC dimer + SO3; C14H13O7S | 325.0389 | 325.0387 | −0.1 | n/a | 3 |
| 4MC dimer + SO3; C14H13O7S | 325.0381 | 325.0387 | 0.7 | n/a | 1 |
| H2S-4MC and SO2-4MC adducts: wine relevant concentrations | |||||
| 4MC + SO3; C7H7O5S | 203.0022 | 203.0020 | −0.2 | 203.0019 | 1 |
| 4MC dimer + SO3; C14H13O7S | 325.0389 | 325.0387 | −0.1 | n/a | 5 |
| 4MC dimer + SO3; C14H13O7S | 325.0390 | 325.0387 | −0.2 | n/a | 1 |
| H2S-4MC and SO2-4MC adducts: wine relevant concentrations + metal ions | |||||
| 4MC + SO3; C7H7O5S | 203.0024 | 203.0020 | −0.4 | 203.0019 | 1 |
| 4MC dimer + SO3; C14H13O7S | 325.0389 | 325.0387 | −0.1 | n/a | 2 |
| 4MC dimer + SO3; C14H13O7S | 325.0381 | 325.0387 | 0.7 | n/a | 1 |
| Base Concentration | Spiked Concentration | Averages | Concentration Range | Legal Limits | |||||
|---|---|---|---|---|---|---|---|---|---|
| Verdelho | Shiraz | Verdelho | Shiraz | Model Wine | White Wine 2 | Red Wine 2 | |||
| Cu2+ (mg/L) | 0.026 | 0.015 | 1.01 | 0.99 | 1.00 | 0.15 1 | 0.00–1.89 1 | 1 | 1 |
| Total/free SO2 (mg/L) | <4.00/<4.00 | <4.00/<4.00 | 98.3/87.7 | 60.7/40.8 | 100/99.67 | 74.0 3 | 10.0 > 350 3 | 200 | 150 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekker, M.Z.; Smith, M.E.; Smith, P.A.; Wilkes, E.N. Formation of Hydrogen Sulfide in Wine: Interactions between Copper and Sulfur Dioxide. Molecules 2016, 21, 1214. https://doi.org/10.3390/molecules21091214
Bekker MZ, Smith ME, Smith PA, Wilkes EN. Formation of Hydrogen Sulfide in Wine: Interactions between Copper and Sulfur Dioxide. Molecules. 2016; 21(9):1214. https://doi.org/10.3390/molecules21091214
Chicago/Turabian StyleBekker, Marlize Z., Mark E. Smith, Paul A. Smith, and Eric N. Wilkes. 2016. "Formation of Hydrogen Sulfide in Wine: Interactions between Copper and Sulfur Dioxide" Molecules 21, no. 9: 1214. https://doi.org/10.3390/molecules21091214
APA StyleBekker, M. Z., Smith, M. E., Smith, P. A., & Wilkes, E. N. (2016). Formation of Hydrogen Sulfide in Wine: Interactions between Copper and Sulfur Dioxide. Molecules, 21(9), 1214. https://doi.org/10.3390/molecules21091214