Novel Amino-Pyridine Functionalized Chitosan Quaternary Ammonium Derivatives: Design, Synthesis, and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
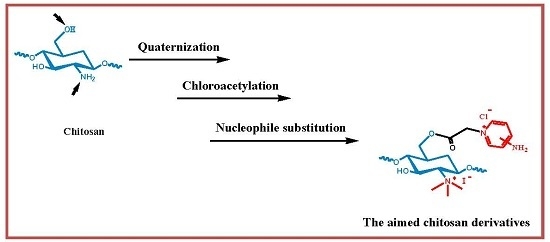
2.1. Chemical Syntheses and Characterization
2.2. Antioxidant Activities
3. Experimental Section
3.1. Materials
3.2. Analytical Methods
3.3. The Synthesis of Chitosan Derivatives
3.4. Antioxidant Ability
3.4.1. Hydroxyl-Radical Scavenging Ability Assay
3.4.2. DPPH-Radical Scavenging Ability Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| DMSO | Dimethyl Sulphoxide |
| DMF | N,N-Dimethylformamide |
| EDTA | Ethylenediaminetetraacetic acid |
| NMP | N-Methyl pyrrolidone |
References
- Peng, Z.; Liu, M.; Fang, Z.; Zhang, Q. In vitro antioxidant effects and cytotoxicity of polysaccharides extracted from Laminaria japonica. Int. J. Biol. Macromol. 2012, 50, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-H.; Hu, T.; Li, Z.-Y.; Huang, Z.-L.; Jiang, J.-G. In vitro antioxidant activities of the polysaccharides from Pleurotus tuber-regium (Fr.) Sing. Food. Chem. 2014, 148, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Cheng, W.; Wei, Y.; Zhang, L. Phosphorylated modification and in vitro antioxidant activity of Radix Hedysari polysaccharide. Glycoconj. J. 2012, 29, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, H.; Dong, F.; Xue, Q.; Wang, G.; Qin, S.; Guo, Z. The influence of the cation of quaternized chitosans on antioxidant activity. Carbohydr. Polym. 2009, 78, 439–443. [Google Scholar] [CrossRef]
- Park, P.-J.; Jung, W.-K.; Nam, K.-S.; Shahidi, F.; Kim, S.-K. Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J. Am. Oil Chem. Soc. 2001, 78, 651–656. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. Eur. Polym. J. 2015, 65, 46–62. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Li, Q.; Tan, W.; Zhang, C.; Gu, G.; Guo, Z. Novel triazolyl-functionalized chitosan derivatives with different chain lengths of aliphatic alcohol substituent: Design, synthesis, and antifungal activity. Carbohydr. Res. 2015, 418, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Wang, F.; Xia, Y.; Chen, X.; Lei, C. Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chem. 2012, 132, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Teli, M.D.; Sheikh, J. Extraction of chitosan from shrimp shells waste and application in antibacterial finishing of bamboo rayon. Int. J. Biol. Macromol. 2012, 50, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.L.; Chen, Y.C.; Tan, F.J. Antioxidative properties of a chitosan–glucose Maillard reaction product and its effect on pork qualities during refrigerated storage. Food Chem. 2011, 124, 589–595. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Kuo, C.-L.; Chen, C.-C. Preparation and important functional properties of water-soluble chitosan produced through Maillard reaction. Bioresour. Technol. 2005, 96, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-B.; Lin, Y.-C.; Chen, H.-H. Low molecular weight chitosan prepared with the aid of cellulase, lysozyme and chitinase: Characterisation and antibacterial activity. Food Chem. 2009, 116, 47–53. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, B.; Zeng, L.; Zhang, Z.; Liu, Y.; Du, Y.; Xiao, L. The physicochemical properties and antitumor activity of cellulase-treated chitosan. Food Chem. 2004, 84, 107–115. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Yu, C.; Li, Q.; Dong, F.; Wang, G.; Guo, Z. Synthesis, characterization, and antioxidant properties of novel inulin derivatives with amino-pyridine group. Int. J. Biol. Macromol. 2014, 70, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Gao, Z.; Qiu, S.; Dong, F.; Guo, Z. Design, synthesis of novel starch derivative bearing 1,2,3-triazolium and pyridinium and evaluation of its antifungal activity. Carbohydr. Polym. 2017, 157, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Dong, F.; Wei, L.; Guo, Z. Synthesis, characterization, and antifungal property of chitosan ammonium salts with halogens. Int. J. Biol. Macromol. 2016, 92, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.-Q.; Xiong, W.-Y.; Wang, H.; Sun, Y.; Liu, H.-Z. Preparation, water solubility and antioxidant activity of branched-chain chitosan derivatives. Carbohydr. Polym. 2011, 83, 1787–1796. [Google Scholar] [CrossRef]
- Bernardino, A.M.; da Silva Pinheiro, L.C.; Rodrigues, C.R.; Loureiro, N.I.; Castro, H.C.; Lanfredi-Rangel, A.; Sabatini-Lopes, J.; Borges, J.C.; Carvalho, J.M.; Romeiro, G.A.; et al. Design, synthesis, SAR, and biological evaluation of new 4-(phenylamino)thieno[2,3-b]pyridine derivatives. Bioorg. Med. Chem. 2006, 14, 5765–5770. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, C.G.; Barresi, V.; Berellini, G.; Musumarra, G. Design and synthesis of trans 2-(furan-2-yl)vinyl heteroaromatic iodides with antitumour activity. Bioorg. Med. Chem. 2008, 16, 4150–4159. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.W.; Im, W.B.; Rhee, J.K.; Shim, M.J.; Kim, W.B.; Choi, E.C. Synthesis and antibacterial activity of oxazolidinones containing pyridine substituted with heteroaromatic ring. Bioorg. Med. Chem. 2004, 12, 5909–5915. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.; Scarpelli, R.; Bollbuck, B.; Werschkun, B.; Pereira, M.; Wartmann, M.; Altmann, K.; Zaharevitz, D.; Gussio, R.; Giannakakou, P. Chemical synthesis and biological properties of pyridine epothilones. Chem. Biol. 2000, 7, 593–599. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Kim, H.S.; Jadhav, J.R.; Jung, S.J.; Kwak, J.H. Synthesis and antimicrobial activity of imidazole and pyridine appended cholestane-based conjugates. Bioorg. Med. Chem. Lett. 2013, 23, 4315–4318. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W. Synthetic methods and applications of chitosan containing pyridylmethyl moiety and its quaternized derivatives: A review. Carbohydr. Polym. 2010, 80, 631–647. [Google Scholar] [CrossRef]
- Li, Q.; Ren, J.; Dong, F.; Feng, Y.; Gu, G.; Guo, Z. Synthesis and antifungal activity of thiadiazole-functionalized chitosan derivatives. Carbohydr. Res. 2013, 373, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Q.; Wang, G.; Dong, F.; Zhou, H.; Zhang, J. Synthesis, characterization, and antifungal activity of novel inulin derivatives with chlorinated benzene. Carbohydr. Polym. 2014, 99, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, Q.; Dong, F.; Feng, Y.; Guo, Z. Phenolic antioxidants-functionalized quaternized chitosan: Synthesis and antioxidant properties. Int. J. Biol. Macromol. 2013, 53, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-Y.; Kim, S.-K. Reactive oxygen species scavenging activity of aminoderivatized chitosan with different degree of deacetylation. Bioorg. Med. Chem. 2006, 14, 5989–5994. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, P.; Subhapradha, N.; Shanmugam, V.; Shanmugam, A. Extraction, characterization and antioxidant property of chitosan from cuttlebone Sepia kobiensis (Hoyle 1885). Int. J. Biol. Macromol. 2014, 64, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry, Part A: Structrue and Mechanisms, 5th ed.; Springer: Beijing, China, 2009; pp. 793–794. [Google Scholar]
- El Ashry, E.S.H.; El-Rafey, E.; Rezki, N.; Abou-Elnaga, H.H.; Bakry, W.M.A.; Boghdadi, Y.M. Evaluation of some functionalized imidazoles and 1,2,4-triazoles as antioxidant additives for industrial lubricating oils and correlating the results with the structures of additives using empirical AM1 calculations. J. Saudi Chem. Soc. 2014, 18, 443–449. [Google Scholar] [CrossRef]
- Tan, W.Q.; Li, Q.; Li, W.C.; Dong, F.; Guo, Z.Y. Synthesis and antioxidant property of novel 1,2,3-triazole-linkedstarch derivatives via ‘click chemistry’. Int. J. Biol. Macromol. 2016, 82, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Snyman, D.; Hamman, J.H.; Kotze, J.S.; Rollings, J.E.; Kotze, A.F. The relationship between the absolute molecular weight and the degree of quaternisation of N-trimethyl chitosan chloride. Carbohydr. Polym. 2002, 50, 145–150. [Google Scholar] [CrossRef]
- Pasanphan, W.; Buettner, G.R.; Chirachanchai, S. Chitosan conjugated with deoxycholic acid and gallic acid: A novel biopolymer-based additive antioxidant for polyethylene. J. Appl. Polym. Sci. 2008, 109, 38–46. [Google Scholar] [CrossRef]
- Guo, Z.; Peng, L. A characterization of band-limited function and its applications. Sci. Sin. Math. 2014, 44, 469–476. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhang, C.; Tan, W.; Gu, G.; Guo, Z. Novel Amino-Pyridine Functionalized Chitosan Quaternary Ammonium Derivatives: Design, Synthesis, and Antioxidant Activity. Molecules 2017, 22, 156. https://doi.org/10.3390/molecules22010156
Li Q, Zhang C, Tan W, Gu G, Guo Z. Novel Amino-Pyridine Functionalized Chitosan Quaternary Ammonium Derivatives: Design, Synthesis, and Antioxidant Activity. Molecules. 2017; 22(1):156. https://doi.org/10.3390/molecules22010156
Chicago/Turabian StyleLi, Qing, Caili Zhang, Wenqiang Tan, Guodong Gu, and Zhanyong Guo. 2017. "Novel Amino-Pyridine Functionalized Chitosan Quaternary Ammonium Derivatives: Design, Synthesis, and Antioxidant Activity" Molecules 22, no. 1: 156. https://doi.org/10.3390/molecules22010156
APA StyleLi, Q., Zhang, C., Tan, W., Gu, G., & Guo, Z. (2017). Novel Amino-Pyridine Functionalized Chitosan Quaternary Ammonium Derivatives: Design, Synthesis, and Antioxidant Activity. Molecules, 22(1), 156. https://doi.org/10.3390/molecules22010156