Effect of Film-Forming Alginate/Chitosan Polyelectrolyte Complex on the Storage Quality of Pork
Abstract
:1. Introduction
2. Results and Discussion
2.1. First Part of Experiment—Analysis of Hydrosols
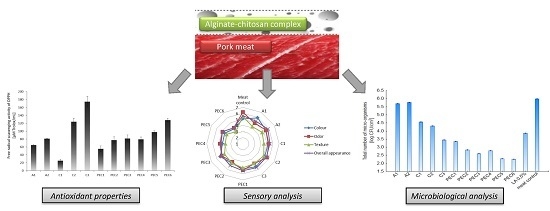
Free Radical Scavenging Activity of DPPH (2,2-Diphenyll-picrylhydrazyl) and Ferric Reducing Antioxidant Power (FRAP) of Experimental Hydrosols
2.2. Second Part of Experiment—Application Analysis
2.2.1. Total Antioxidant Capacity (TAC) Measurements
2.2.2. Microbiological Analysis
2.2.3. Sensory Analysis
3. Materials and methods
3.1. Chemicals
3.2. Hydrosols Preparation
3.3. First Part of the Experiment—The Analysis of Hydrosols
3.3.1. DPPH Radical Scavenging Activity
3.3.2. Ferric Reducing Antioxidant Power—FRAP
3.4. Second Part of the Experiment—The Application Analysis
3.4.1. Meat Samples Preparation
3.4.2. Total Antioxidant Capacity (TAC) Measurements
3.4.3. Microbiological Analysis
3.4.4. Sensory Analysis
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| A | Sodium alginate |
| C | Chitosan |
| CFU | Colonies-forming unit |
| DPPH | 2,2-Diphenyll-picrylhydrazyl |
| E | Enterobacteriaceae |
| FRAP | Ferric reducing antioxidant power |
| HPMC | Hydroxypropyl methyl cellulose |
| LA | Lactic acid |
| LAB | lactic acid bacteria |
| LPS | Lipopolysaccharide |
| MAP | Modified atmosphere packaging |
| MRPs | Maillard Reaction Products |
| MRS | de Man, Rogosa and Sharpe agar |
| P | Psychrotrophs |
| PAA | Poly(acrylic acid) |
| PEC | Polyelectrolyte complex |
| SEM | Scanning electron microscope |
| TAC | Total antioxidant capacity |
| TBARS | Thiobarbituric acid reactive substances |
| TNM | Total number of micro-organisms |
| TPTZ | 2,4,6-Tripyridyls-triazine |
| VRBG | Violet red bile glucose agar |
| YM | Yeast and molds |
References and Notes
- Dai, Y.; Lu, Y.; Wu, W.; Lu, X.; Han, Z.; Liu, Y.; Li, X.; Dai, R. Changes in oxidation, color and texture deteriorations during refrigerated storage of ohmically and water bath-cooked pork meat. Innov. Food Sci. Emerg. Technol. 2014, 26, 341–346. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Sabow, A.B.; Sazili, A.Q.; Zulkifli, I.; Goh, Y.M.; Ab Kadir, M.Z.; Abdulla, N.R.; Nakyinsige, K.; Kaka, U.; Adeyemi, K.D. A comparison of bleeding efficiency, microbiological quality and lipid oxidation in goats subjected to conscious halal slaughter and slaughter following minimal anesthesia. Meat Sci. 2015, 104, 78–84. [Google Scholar] [PubMed]
- Fung, D.Y. Microbial hazards in food: Food-borne infections and intoxications. In Handbook of Meat Processing; Toldra, F., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2010; pp. 481–500. [Google Scholar]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage-interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef]
- Leistner, L. Basic aspect of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef]
- Singh, S.; Shalini, R. Effect of Hurdle Technology in Food Preservation: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kamil, J.Y.; Shahidi, F. Chitosan as an edible invisible film for quality preservation of herring and Atlantic cod. J. Agric. Food Chem. 2002, 50, 5167–5178. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.P.; Mancini, R.A.; Joseph, P.; Ramanathan, R.; Konda, M.K.; Dady, G.; Yin, S. Packaging-specific influence of chitosan on color stability and lipid oxidation in refrigerated ground beef. Meat Sci. 2010, 86, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Zimoch-Korzycka, A.; Rouilly, A.; Bobak, Ł.; Jarmoluk, A.; Korzycki, M. Chitosan and Cystatin/Lysozyme Preparation as Protective Edible Films Components. Int. J. Polym. Sci. 2015, 2015, 139617. [Google Scholar] [CrossRef]
- Soultos, N.; Tzikas, Z.; Abrahim, A.; Georgantelis, D.; Ambrosiadis, I. Chitosan effects on quality properties of Greek style fresh pork sausages. Meat Sci. 2008, 80, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Wanstedt, K.G.; Seideman, S.C.; Connelly, L.S.; Quenzer, N.M. Sensory attributes of precooked, calcium alginate-coated pork patties. J. Food Prot. 1981, 44, 732–735. [Google Scholar] [CrossRef]
- Chidanandaiah; Keshri, R.C.; Sanyal, M.K. Effect of sodium alginate coating with preservatives on the quality of meat patties during refrigerated (4 ± 1 °C) storage. J. Muscle Foods 2009, 20, 275–292. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Zimoch-Korzycka, A.; Kulig, D.; Jarmoluk, A.; Marycz, K.; Matuszczak, W. Study of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges Formation Process. Polymers 2016, 8, 8. [Google Scholar] [CrossRef]
- Trung, T.S.; Bao, H.N.D. Physicochemical Properties and Antioxidant Activity of Chitin and Chitosan Prepared from Pacific White Shrimp Waste. Int. J. Carbohydr. Chem. 2015, 2015, 706259. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan chemistry: Relevance to the biomedical science. Adv. Polym. Sci. 2005, 186, 151–209. [Google Scholar]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Xue, C.; Yu, G.; Hirata, T.; Terao, J.; Lin, H. Antioxidative activities of several marine polysaccharides evaluated in a phosphatidylcholine-liposomal suspension and organic solvents. Biosci. Biotechnol. Biochem. 1998, 62, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chou, C.C. Antioxidative activities of water-soluble disaccharide chitosan derivatives. Food Res. Int. 2004, 37, 883–889. [Google Scholar] [CrossRef]
- Kamil, J.Y.V.A.; Jeon, Y.J.; Shahidi, F. Antioxidative activity of chitosans of different viscosity in cooked comminuted flesh of herring (Clupea harengus). Food Chem. 2002, 79, 69–77. [Google Scholar] [CrossRef]
- Serpen, A.; Capuano, E.; Fogliano, V.; Gökmen, V. A new procedure to measure the antioxidant activity of insoluble food components. J. Agric. Food Chem. 2007, 55, 7676–7681. [Google Scholar] [CrossRef] [PubMed]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A. Cross-linked alginate/chitosan polyelectrolytes as carrier of active compound and beef color stabilizer. Meat Sci. 2016, 123, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Zalloum, H.M.; Mubarak, M.S. Antioxidant Polymers: Metal Chelating Agents. In Antioxidant Polymers: Synthesis, Properties, and Applications; Cirilo, G., Iemma, F., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012; pp. 87–114. [Google Scholar]
- Love, J.D.; Pearson, A.M. Lipid oxidation in meat and meat products—A review. J. Am. Oil Chem. Soc. 1971, 48, 547–549. [Google Scholar] [CrossRef]
- Anonymous: Commission Regulation (EC) No 2073/2005 of 15 November 2005, on microbiological criteria for foodstuffs. OJEU 2005, l35:1.
- Kröckel, L. The Role of Lactic Acid Bacteria in Safety and Flavour Development of Meat and Meat Products. In Lactic Acid Bacteria—R&D for Food, Health and Livestock Purposes; Kongo, J.M., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Ulbin-Figlewicz, N.; Zimoch-Korzycka, A.; Jarmoluk, A. Antibacterial Activity and Physical Properties of Edible Chitosan Films Exposed to Low-pressure Plasma. Food Bioprocess Technol. 2014, 7, 3646–3654. [Google Scholar] [CrossRef]
- Zimoch-Korzycka, A.; Jarmoluk, A. The use of chitosan, lysozyme, and the nano-silver as antimicrobial ingredients of edible protective hydrosols applied into the surface of meat. J. Food Sci. Technol. 2014, 52, 5996–6002. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A Review of the antimicrobial activity of chitosan. Polím. Ciênc. Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 2011, 22, 608–615. [Google Scholar] [CrossRef]
- Yang, M.Y.; Lin, H.T.; Wu, T.H.; Chen, C.C. Wettability and Antibacterial Assessment of Chitosan Containing Radiation-Induced Graft Nonwoven Fabric of Polypropylene-g-Acrylic Acid. J. Appl. Polym. Sci. 2003, 90, 1331–1336. [Google Scholar] [CrossRef]
- Ortega-Ortiz, H.; Gutiérrez-Rodríguez, B.; Cadenas-Pliego, G.; Jimenez, L.I. Antibacterial activity of chitosan and the interpolyelectrolyte complexes of poly(acrylic acid)-chitosan. Braz. Arch. Biol. Technol. 2010, 53, 623–628. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial Edible Films and Coatings for Meat and Meat Products Preservation. Sci. World J. Artic. 2014. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.D.; Ji, D.Y.; Chang, W.J.; Yang, J.C.; Lee, S.Y. Chitosan-based polyelectrolyte complex scaffolds with antibacterial properties for treating dental bone defects. Mater. Sci. Eng. C 2012, 32, 207–214. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal Activity of Chitosan Nanoparticles and Correlation with Their Physical Properties. Int. J. Biomater. 2012. [Google Scholar] [CrossRef] [PubMed]
- Séon, L.; Lavalle, P.; Schaaf, P.; Boulmedais, F. Polyelectrolyte Multilayers: A Versatile Tool for Preparing Antimicrobial Coatings. Langmuir 2015, 31, 12856–12872. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Yeh, J.Y.; Chen, P.C.; Hsu, C.K. Phenolic content and DPPH radical scavenging activity of yam-containing surimi gels influenced by salt and heating. Asian J. Health Inf. Sci. 2007, 2, 1–11. [Google Scholar]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Microbiology of Food and Animal Feeding Stuffs—Horizontal Methods for Sampling Techniques from Surfaces Using Contact Plates and Swabs; ISO 18593:2004; ISO: Geneva, Switzerland, 2004.
- Microbiology of Food and Animal Feeding Stuffs—Carcass Sampling for Microbiological Analysis; ISO 17604:2003; ISO: Geneva, Switzerland, 2003.
- Meat and Meat Products—Enumeration of Microorganisms—Colony Count Technique at 30 °C; ISO 2293:1988; ISO: Geneva, Switzerland, 1988.
- Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms; ISO 17410:2001; ISO: Geneva, Switzerland, 2001.
- General Guidance for enumeration of yeasts and moulds—Colony Count Technique at 25°C; ISO 21527-1:2008; ISO: Geneva, Switzerland, 2008.
- Meat and Meat Products—Enumeration of Lactic acid Bacteria—Colony-Count Technique at 30 °C; ISO 13721:1995; ISO: Geneva, Switzerland, 1995.
- Meat and Meat Products—Detection and Enumeration of Enterobacteriaceae without Resuscitation—MPN Technique and Colony Count Technique; ISO 5552:2005; ISO: Geneva, Switzerland, 2005.
- Sample Availability: Not available.




| Variant | Storage Time (Days) | DPPH | FRAP | ||
|---|---|---|---|---|---|
| Raw Meat | Cooked Meat | Raw Meat | Cooked Meat | ||
| Meat control | 0 | 15.38 ± 0.14 hi | 15.06 ± 0.39 i−l | 11.97 ± 0.41 ab | 9.43 ± 0.19 bc |
| A1 | 13.08 ± 0.18 a–c | 12.61 ± 0.22 a–c | 17.85 ± 0.64 e–h | 14.26 ± 0.15 k | |
| A2 | 14.77 ± 0.04 e–h | 13.74 ± 0.12 d–h | 18.06 ± 0.29 e–i | 16.14 ± 0.19 h | |
| C1 | 13.98 ± 0.08 c–f | 12.76 ± 0.11 a–d | 10.77 ± 0.31 a | 13.30 ± 0.18 ij | |
| C2 | 14.24 ± 0.01 d–g | 13.77 ± 0.22 d–h | 11.96 ± 0.13 ab | 12.62 ± 0.60 hi | |
| C3 | 15.01 ± 0.13 f–h | 14.55 ± 0.11 g–k | 16.61 ± 0.35 e | 13.54 ± 0.05 jk | |
| PEC1 | 13.04 ± 0.68 a–c | 12.98 ± 0.53 b–e | 11.63 ± 1.05 ab | 11.28 ± 0.17 fg | |
| PEC2 | 12.63 ± 1.01 ab | 12.33 ± 0.60 ab | 16.68 ± 0.45 e | 11.65 ± 0.24 g | |
| PEC3 | 16.22 ± 0.22 i | 13.58 ± 0.05 c–g | 14.60 ± 0.96 d | 14.17 ± 0.48 k | |
| PEC4 | 12.17 ± 0.35 a | 11.95 ± 0.21 a | 12.15 ± 0.40 ab | 12.70 ± 0.24 hi | |
| PEC5 | 12.80 ± 0.39 ab | 13.10 ± 0.22 b–f | 14.68 ± 1.62 d | 15.09 ± 0.45 l | |
| PEC6 | 14.17 ± 0.06 d–f | 13.89 ± 0.08 e–h | 14.38 ± 0.40 cd | 15.30 ± 0.18 lm | |
| Meat control | 7 | 15.58 ± 0.42 hi | 17.58 ± 0.36 op | 13.09 ± 0.15 bc | 8.90 ± 0.15 b |
| A1 | 15.27 ± 0.01 g–i | 15.28 ± 0.18 j–l | 17.42 ± 0.14 e–g | 20.14 ± 0.10 su | |
| A2 | 18.50 ± 0.24 k | 19.37 ± 0.16 rs | 19.30 ± 0.15 h–j | 21.38 ± 0.11 t | |
| C1 | 13.88 ± 0.03 c–e | 14.11 ± 0.09 f–i | 17.18 ± 0.12 ef | 9.84 ± 0.11 cd | |
| C2 | 14.54 ± 0.13 e–h | 14.71 ± 0.09 h–k | 17.79 ± 0.03 e–h | 10.68 ± 0.05 ef | |
| C3 | 15.53 ± 0.06 hi | 16.00 ± 0.26 lm | 19.85 ± 0.12 jk | 12.06 ± 0.30 gh | |
| PEC1 | 14.08 ± 0.04 c–f | 13.92 ± 0.12 e–h | 19.54 ± 0.09 ij | 11.79 ± 0.26 g | |
| PEC2 | 14.55 ± 0.59 e–h | 15.52 ± 0.26 kl | 21.74 ± 0.20 l | 19.07 ± 0.02 qr | |
| PEC3 | 16.21 ± 0.45 i | 16.69 ± 0.19 m–o | 21.61 ± 0.27 l | 20.98 ± 0.05 t | |
| PEC4 | 13.35 ± 0.28 b–d | 14.43 ± 0.13 g–j | 21.34 ± 0.05 kl | 16.43 ± 0.32 no | |
| PEC5 | 15.55 ± 0.66 hi | 14.79 ± 0.11 h–k | 21.28 ± 0.50 kl | 18.66 ± 0.24 q | |
| PEC6 | 17.31 ± 0.07 j | 16.50 ± 0.14 mn | 23.74 ± 0.36 mn | 21.57 ± 0.16 t | |
| Meat control | 14 | 17.56 ± 0.43 j | 17.49 ± 0.27 op | 12.94 ± 0.13 bc | 7.90 ± 0.03 a |
| A1 | 19.27 ± 0.06 kl | 14.97 ± 0.62 i–k | 24.11 ± 0.41 n | 20.98 ± 0.05 tu | |
| A2 | 21.24 ± 0.09 n | 19.77 ± 1.03 rs | 22.52 ± 0.43 lm | 21.19 ± 0.21 t | |
| C1 | 20.71 ± 0.07 mn | 19.00 ± 0.03 qr | 16.86 ± 0.33 ef | 9.35 ± 0.23 bc | |
| C2 | 22.57 ± 0.25 o | 20.28 ± 0.10 st | 12.95 ± 0.12 bc | 10.38 ± 0.45 de | |
| C3 | 24.06 ± 0.06 p | 21.05 ± 0.16 t | 18.36 ± 0.89 f–j | 15.95 ± 0.28 mn | |
| PEC1 | 18.86 ± 0.33 kl | 17.39 ± 0.13 n–p | 14.76 ± 0.69 d | 17.64 ± 0.82 p | |
| PEC2 | 19.78 ± 0.17 lm | 19.00 ± 0.24 qr | 17.16 ± 0.35 ef | 17.15 ± 0.36 op | |
| PEC3 | 20.38 ± 0.36 mn | 20.89 ± 0.26 t | 19.92 ± 0.08 jk | 21.22 ± 0.05 t | |
| PEC4 | 19.72 ± 0.09 lm | 13.77 ± 0.59 d–h | 18.80 ± 0.27 g–j | 19.65 ± 0.17 rs | |
| PEC5 | 20.99 ± 0.03 n | 18.35 ± 0.37 pq | 22.51 ± 0.54 lm | 20.93 ± 0.18 t | |
| PEC6 | 23.13 ± 0.42 op | 19.83 ± 0.03 rs | 24.22 ± 0.10 n | 21.69 ± 0.12 t | |
| Variant | Storage Time (Days) | TVC | YM | P | LAB | Enterobacteriaceae |
|---|---|---|---|---|---|---|
| LA | 0 | 3.03 ± 0.03 e | 1.28 ± 0.03 b–e | 2.03 ± 0.04 b | 1.64 ± 0.03 c | nd |
| Meat control | 3.87 ± 0.02 i | 2.26 ± 0.02 i | 3.87 ± 0.02 g | 1.70 ± 0.01 d | ||
| A1 | 3.79 ± 0.01 i | 2.15 ± 0.02 hi | 3.63 ± 0.03 f | nd | ||
| A2 | 3.74 ± 0.01 hi | 2.04 ± 0.02 g–i | 3.59 ± 0.03 f | nd | ||
| C1 | 3.67 ± 0.00 h | 1.73 ± 0.09 f–h | 3.11 ± 0.05 e | 1.47 ± 0.02 b | ||
| C2 | 3.51 ± 0.00 g | 1.69 ± 0.08 e–g | 2.35 ± 0.01 c | 1.06 ± 0.05 a | ||
| C3 | 3.37 ± 0.02 f | 1.52 ± 0.02 d–f | 2.16 ± 0.05 b | nd | ||
| PEC1 | 2.98 ± 0.01 e | 1.07 ± 0.18 a–c | 2.34 ± 0.05 c | nd | ||
| PEC2 | 2.68 ± 0.11 d | 1.19 ± 0.02 a–e | 2.93 ± 0.06 d | nd | ||
| PEC3 | 2.36 ± 0.04 c | 1.04 ± 0.15 ab | 2.37 ± 0.12 c | nd | ||
| PEC4 | 2.70 ± 0.02 d | 0.80 ± 0.39 a | 1.64 ± 0.02 a | nd | ||
| PEC5 | 2.05 ± 0.04 b | 1.50 ± 0.02 c–f | 1.61 ± 0.03 a | nd | ||
| PEC6 | 1.93 ± 0.02 a | 1.38 ± 0.39 b–f | 2.95 ± 0.05 d | nd | ||
| LA | 7 | 3.64 ± 0.02 f | 2.75 ± 0.04 f | 2.20 ± 0.05 c | 3.01 ± 0.09 f | nd |
| Meat control | 6.38 ± 0.08 k | 3.36 ± 0.06 h | 4.46 ± 0.02 i | 4.26 ± 0.01 h | ||
| A1 | 5.57 ± 0.01 i | 3.12 ± 0.06 g | 4.40 ± 0.04 i | 3.95 ± 0.04 g | ||
| A2 | 5.89 ± 0.06 j | 3.03 ± 0.01 g | 4.33 ± 0.06 i | 4.15 ± 0.04 h | ||
| C1 | 4.10 ± 0.03 h | 2.19 ± 0.01 e | 3.50 ± 0.02 h | 2.97 ± 0.01 f | ||
| C2 | 3.90 ± 0.01 g | 2.09 ± 0.05 de | 2.66 ± 0.04 f | 2.49 ± 0.00 e | ||
| C3 | 3.16 ± 0.00 e | 1.66 ± 0.02 b | 2.28 ± 0.06 cd | 2.10 ± 0.05 d | ||
| PEC1 | 3.13 ± 0.04 e | 1.89 ± 0.05 c | 2.49 ± 0.01 e | 1.46 ± 0.10 b | ||
| PEC2 | 2.92 ± 0.01 d | 1.51 ± 0.10 a | 2.91 ± 0.04 g | 1.26 ± 0.15 a | ||
| PEC3 | 2.55 ± 0.01 b | 1.95 ± 0.04 cd | 2.69 ± 0.10 f | 1.76 ± 0.03 c | ||
| PEC4 | 2.72 ± 0.00 c | 1.50 ± 0.02 a | 1.72 ± 0.05 a | 1.62 ± 0.00 bc | ||
| PEC5 | 2.21 ± 0.05 a | 2.03 ± 0.04 cd | 1.93 ± 0.05 b | 1.72 ± 0.01 c | ||
| PEC6 | 2.19 ± 0.01 a | 1.92 ± 0.07 c | 2.38 ± 0.02 de | 2.36 ± 0.03 e | ||
| LA | 14 | 4.93 ± 0.07 e | 4.08 ± 0.03 e | 2.61 ± 0.05 cd | 4.90 ± 0.01 j | nd |
| Meat control | 7.86 ± 0.03 i | 4.21 ± 0.04 e | 6.31 ± 0.02 h | 6.18 ± 0.02 m | ||
| A1 | 7.74 ± 0.01 h | 4.14 ± 0.02 e | 5.56 ± 0.04 g | 5.47 ± 0.02 k | ||
| A2 | 7.70 ± 0.03 h | 4.13 ± 0.02 e | 5.49 ± 0.04 g | 5.51 ± 0.00 l | ||
| C1 | 5.91 ± 0.00 g | 3.43 ± 0.19 d | 3.82 ± 0.05 f | 3.70 ± 0.01 i | ||
| C2 | 5.54 ± 0.02 f | 3.23 ± 0.05 c | 2.79 ± 0.06 d | 3.29 ± 0.05 h | ||
| C3 | 3.82 ± 0.09 c | 2.65 ± 0.05 b | 2.56 ± 0.02 bc | 3.15 ± 0.01 g | ||
| PEC1 | 3.96 ± 0.00 d | 2.66 ± 0.03 b | 2.56 ± 0.00 bc | 2.28 ± 0.01 c | ||
| PEC2 | 2.91 ± 0.01 b | 2.16 ± 0.03 a | 3.06 ± 0.20 e | 2.17 ± 0.00 b | ||
| PEC3 | 2.90 ± 0.03 b | 2.21 ± 0.06 a | 3.25 ± 0.07 e | 2.56 ± 0.00 d | ||
| PEC4 | 2.95 ± 0.01 b | 2.31 ± 0.02 a | 1.97 ± 0.03 a | 2.60 ± 0.00 e | ||
| PEC5 | 2.62 ± 0.02 a | 2.33 ± 0.01 a | 2.07 ± 0.02 a | 2.05 ± 0.01 a | ||
| PEC6 | 2.65 ± 0.01 a | 2.16 ± 0.06 a | 2.38 ± 0.01 b | 2.69 ± 0.01 f |
| Coding | Polymers | A/C Mutual Mass Ratio | ||
|---|---|---|---|---|
| A (%) | C (%) | |||
| Controls | A1 | 0.30 | 0 | - |
| A2 | 0.60 | 0 | ||
| C1 | 0 | 0.50 | ||
| C2 | 0 | 0.75 | ||
| C3 | 0 | 1.00 | ||
| Polyelectrolyte complex | PEC1 | 0.30 | 0.50 | 0.60 |
| PEC2 | 0.30 | 0.75 | 0.40 | |
| PEC3 | 0.30 | 1.00 | 0.30 | |
| PEC4 | 0.60 | 0.50 | 1.20 | |
| PEC5 | 0.60 | 0.75 | 0.80 | |
| PEC6 | 0.60 | 1.00 | 0.60 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulig, D.; Zimoch-Korzycka, A.; Król, Ż.; Oziembłowski, M.; Jarmoluk, A. Effect of Film-Forming Alginate/Chitosan Polyelectrolyte Complex on the Storage Quality of Pork. Molecules 2017, 22, 98. https://doi.org/10.3390/molecules22010098
Kulig D, Zimoch-Korzycka A, Król Ż, Oziembłowski M, Jarmoluk A. Effect of Film-Forming Alginate/Chitosan Polyelectrolyte Complex on the Storage Quality of Pork. Molecules. 2017; 22(1):98. https://doi.org/10.3390/molecules22010098
Chicago/Turabian StyleKulig, Dominika, Anna Zimoch-Korzycka, Żaneta Król, Maciej Oziembłowski, and Andrzej Jarmoluk. 2017. "Effect of Film-Forming Alginate/Chitosan Polyelectrolyte Complex on the Storage Quality of Pork" Molecules 22, no. 1: 98. https://doi.org/10.3390/molecules22010098
APA StyleKulig, D., Zimoch-Korzycka, A., Król, Ż., Oziembłowski, M., & Jarmoluk, A. (2017). Effect of Film-Forming Alginate/Chitosan Polyelectrolyte Complex on the Storage Quality of Pork. Molecules, 22(1), 98. https://doi.org/10.3390/molecules22010098