Solvent-Free Synthesis and Safener Activity of Sulfonylurea Benzothiazolines
Abstract
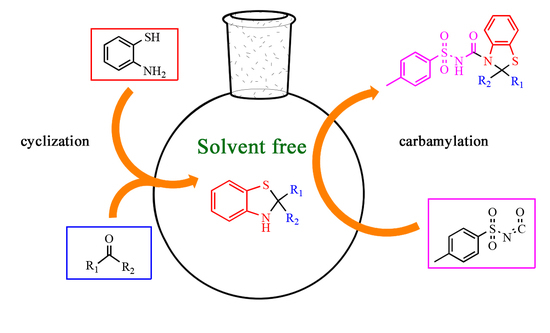
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Activity
3. Materials and Methods
3.1. Reagents and Analysis
3.2. General Procedure for the Preparation of 1,3-Benzothiazoline Derivatives 2
3.3. General Procedure for the Preparation of Sulfonylurea Benzothiazoline Derivatives 3
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berrade, L.; Aisa, B.; Ramirez, M.J.; Galiano, S.; Guccione, S.; Moltzau, L.R.; Levy, F.O.; Nicoletti, F.; Battaglia, G.; Molinaro, G.; et al. Novel benzo [b] thiophene derivatives as new potential antidepressants with rapid onset of action. J. Med. Chem. 2011, 54, 3086–3090. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, A.; Tondi, D.; Cancian, L.; Morandi, F.; Cannazza, G.; Segatore, B.; Prati, F.; Amicosante, G.; Shoichet, B.K.; Costi, M.P. Optimizing cell permeation of an antibiotic resistance inhibitor for improved efficacy. J. Med. Chem. 2007, 50, 5644–5654. [Google Scholar] [CrossRef] [PubMed]
- Mahran, M.; El-Nassry, S.; Allam, S. Synthesis of some new benzothiazole derivatives as potential antimicrobial and antiparasitic agents. Int. J. Pharm. Sci. 2003, 58, 527–530. [Google Scholar] [CrossRef]
- Collier, P.; Ramsey, A.; Austin, P. Growth inhibitory and biocidal activity of some isothiazolone biocides. J. Appl. Microbiol. 1990, 69, 569–577. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry; Sacher, R.M.; Lee, L.F.; Schafer, D.E.; Howe, R.K. Pesticide Chemistry: Human Welfare and Environment: Synthesis and Structure-activity Relationships; Elsevier Ltd.: Amsterdam, The Netherlands, 1983; pp. 165–168. [Google Scholar]
- Rendina, A.; Campopiano, O.; Marsilii, E.; Hixon, M.; Chi, H.; Taylor, W.; Hagenah, J. Overlap between herbicidal inhibitors of acetyl-coenzyme a carboxylase: Enhanced binding of cyclic triketones, a novel class of graminicide. Pestic. Sci. 1995, 43, 368–371. [Google Scholar]
- Chen, J.L.; Tang, W.; Che, J.Y.; Chen, K.; Yan, G.; Gu, Y.C.; Shi, D.Q. Synthesis and biological activity evaluation of novel α-amino phosphonate derivatives containing a pyrimidinyl moiety as potential herbicidal agents. J. Agric. Food Chem. 2015, 63, 7219–7229. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chi, H.W.; Guan, A.Y.; Liu, C.L.; Ma, H.J.; Cui, D.L. Design, synthesis, and herbicidal activity of novel substituted 3-(pyridin-2-yl) benzenesulfonamide derivatives. J. Agric. Food Chem. 2014, 62, 12491–12496. [Google Scholar] [CrossRef] [PubMed]
- Hatzios, K.K. Herbicide antidotes: Development, chemistry, and mode of action. Adv. Agron. 1983, 36, 265–316. [Google Scholar]
- Komives, T.; Hatzios, K.K. Chemistry and structure-activity relationships of herbicide safeners. Z. Naturforsch. C 1991, 46, 798–804. [Google Scholar]
- Fu, Y.; Qu, L.H.; Zhang, S.S.; Ye, F.; Zhao, L.X.; Gao, S.; Xing, Z.Y. Simple and efficient synthesis of novel N-dichloroacetyl-3,4-dihydro-2H-1,4-benzoxazines. Heterocycl. Commun. 2012, 18, 143–146. [Google Scholar] [CrossRef]
- Fu, Y.; Kang, J.X.; Wang, Y.K.; Liu, J.; Zhao, L.X.; Gao, S.; Ye, F. Design, synthesis and biological activity of novel sulfonylurea oxazolidines. Heterocycles 2016, 92, 740–750. [Google Scholar]
- Ye, F.; Wu, S.L.; Zhao, L.X.; Qu, H.T.; Gao, S.; Fu, Y. Synthesis and safener activity of novel substituted 4-phenoxyacetyl-1,4-benzoxazines. Heterocycles 2015, 91, 1256–1268. [Google Scholar]
- Liso, G.; Trapani, G.; Latrofa, A.; Marchini, P. Oxidative ring-expansion of benzothiazolines into 1,4-benzothiazines. J. Heterocyclic Chem. 1981, 18, 279–282. [Google Scholar] [CrossRef]
- Prakash, G.S.; Mathew, T.; Panja, C.; Vaghoo, H.; Venkataraman, K.; Olah, G.A. Efficient one-pot synthesis of fluorinated benzimidazolines, benzothiazolines, benzoxazolines, and dihydrobenzoxazinones using gallium(III) triflate as a catalyst. Org. Lett. 2007, 9, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Chen, X.; Zhang, Y. Low-valent titanium induced simultaneous reduction of nitro group and SS bond in nitrodisulfides: A novel method for the synthesis of benzothiazoline, benzothiazoles and 2,3-dihydro-1,5-benzothiazepines. Synth. Commun. 2000, 30, 4451–4460. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhong, W.H.; Zhang, Y.M. Simultaneous reduction of nitro group and S-S bond in nitrodisulfides by samarium diiodide: A new approach to benzothiazolines. Chin. Chem. Lett. 2000, 11, 387–388. [Google Scholar]
- Tanaka, K. Solvent-Free Organic Synthesis; Wiley-VCH: New York, NY, USA, 2003. [Google Scholar]
- Kodomari, M.; Satoh, A.; Nakano, R.; Aoyama, T. Solvent-Free Synthesis of benzothiazolines in the presence of alumina. Synth. Commun. 2007, 37, 3329–3335. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors’ lab. |






| Entry | R1 | R2 | Compound 2 | Compound 3 | ||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | Time (h) | Yield (%) # | T (°C) | Time (h) | Yield (%) # | |||
| a | CH3 | CH3 | r.t. | 0.5 | 82 | 12 | 12 | 96 |
| b | CH3 | CH2CH3 | r.t. | 0.5 | 83 | r.t. | 5 | 65 |
| c | CH3 | CH2CH2CH3 | r.t. | 0.5 | 92 | r.t. | 5 | 78 |
| d | CH3 | CH(CH3)2 | 80 | 1 | 84 | r.t. | 12 | 70 |
| e | CH3 | CH2COCH3 | 50 | 3 | 78 | r.t. | 10 | 55 |
| f | CH3 | CH2CH2CH2CH3 | r.t. | 2 | 76 | r.t. | 10 | 50 |
| g | CH3 | CH2CH(CH3)2 | r.t. | 0.5 | 76 | r.t. | 12 | 36 |
| h | CH3 | C(CH3)3 | 88 | 1 | 80 | 36 | 2 | 59 |
| i | CH3 | PhCH2CH2 | r.t. | 0.5 | 86 | 12 | 12 | 95 |
| j | CH2CH3 | CH2CH3 | r.t. | 0.5 | 82 | 5 | 5 | 40 |
| k | CH2CH2CH3 | CH2CH2CH3 | 80 | 0.5 | 56 | 4 | 4 | 82 |
| l | (CH2)4 | r.t. | 0.5 | 91 | 12 | 12 | 95 | |
| m | (CH2)5 | r.t. | 0.5 | 92 | 12 | 12 | 99 | |
| Compound | Root Length Recovery Rate (%) | Plant Height Recovery Rate (%) | Root Fresh Weight Recovery Rate (%) | Plant Fresh Weight Recovery Rate (%) |
|---|---|---|---|---|
| AD-67 | 32.66 efg | 81.62 de | 81.25 b | 89.47 c |
| 3a | 38.05 cde | 68.57 f | 37.50 h | 57.89 ef |
| 3b | 35.02 def | 54.41 g | 43.75 g | 21.05 h |
| 3c | 55.89 a | 109.56 a | 87.50 b | 126.31 a |
| 3d | 42.76 bc | 100.92 ab | 6.25 m | 52.63 f |
| 3e | 19.87 i | 20.59 i | 56.25 e | 42.11 g |
| 3f | 56.23 a | 91.73b cd | 12.50 l | 73.68 d |
| 3g | 27.95 gh | 81.86 de | 37.50 h | 84.21 c |
| 3h | 45.12 b | 72.24 ef | 87.50 b | 105.26 b |
| 3i | 32.66 efg | 84.01 cd | 31.25 i | 110.53 b |
| 3j | 18.18 ij | 73.35 ef | 156.25 a | 57.89 ef |
| 3k | 31.31 fg | 40.26 h | 37.50 h | 57.89 ef |
| 3l | 33.00 efg | 89.34 cd | 18.75 k | 84.21 c |
| 3m | 25.93 h | 92.65 bc | 12.50 l | 110.53 b |
| Compounds | log p a | pKa b | MW | Electronegativity c |
|---|---|---|---|---|
| Chlorsulfuron | 2.63 | 4.1 ± 0.4 | 357.77 |  |
| 3c | 4.85 | 4.6 ± 0.4 | 390.52 |  |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Wang, J.-Y.; Zhang, D.; Chen, Y.-F.; Gao, S.; Zhao, L.-X.; Ye, F. Solvent-Free Synthesis and Safener Activity of Sulfonylurea Benzothiazolines. Molecules 2017, 22, 1601. https://doi.org/10.3390/molecules22101601
Fu Y, Wang J-Y, Zhang D, Chen Y-F, Gao S, Zhao L-X, Ye F. Solvent-Free Synthesis and Safener Activity of Sulfonylurea Benzothiazolines. Molecules. 2017; 22(10):1601. https://doi.org/10.3390/molecules22101601
Chicago/Turabian StyleFu, Ying, Jing-Yi Wang, Dong Zhang, Yu-Feng Chen, Shuang Gao, Li-Xia Zhao, and Fei Ye. 2017. "Solvent-Free Synthesis and Safener Activity of Sulfonylurea Benzothiazolines" Molecules 22, no. 10: 1601. https://doi.org/10.3390/molecules22101601
APA StyleFu, Y., Wang, J. -Y., Zhang, D., Chen, Y. -F., Gao, S., Zhao, L. -X., & Ye, F. (2017). Solvent-Free Synthesis and Safener Activity of Sulfonylurea Benzothiazolines. Molecules, 22(10), 1601. https://doi.org/10.3390/molecules22101601