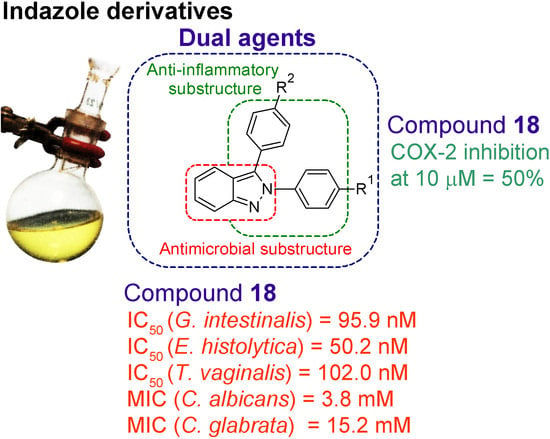
Synthesis and Biological Evaluation of 2H-Indazole Derivatives: Towards Antimicrobial and Anti-Inflammatory Dual Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis
2.2. Antiprotozoal Activity
2.3. Antibacterial and Anticandidal Assays
2.4. In Vitro and In Silico Studies on Cyclooxygenase-2
2.5. Cytotoxicity Assays
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Chemical Synthesis
3.3. Biological Assays
3.3.1. Antiprotozoal Activity Assays
3.3.2. Antibacterial and Anticandidal Assays
3.3.3. Cytotoxicity Assays in Human Cells
3.3.4. Cyclooxygenase Assays
3.4. Molecular Docking
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hodges, K.; Gill, R. Infectious diarrhea: Cellular and molecular mechanisms. Gut Microbes 2010, 1, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Cacciò, S.M.; Sprong, H. Epidemiology of giardiasis in humans. In Giardia: A Model Organism; Luján, H.D., Svärd, S., Eds.; Springer: Vienna, Austria, 2011; pp. 17–28. ISBN 978-3-7091-0198-8. [Google Scholar]
- Ximenez, C.; Partida, O.; Nieves, M.; Hernandez, E.; Moran, P.; Valadez, A.; Gonzalez, E.; Cerritos, R.; Rojas, L. Immune response in human amebiasis: A protective response? In Amebiasis: Biology and Pathogenesis of Entamoeba; Nozaki, T., Bhattacharya, A., Eds.; Springer: Japan, Tokyo, 2015; pp. 497–519. ISBN 978-4-431-55200-0. [Google Scholar]
- Parry, C.M.; Hien, T.T.; Dougan, G.; White, N.J.; Farrar, J.J. Typhoid fever. N. Eng. J. Med. 2002, 347, 1770–1782. [Google Scholar] [CrossRef] [PubMed]
- Upcroft, P.; Upcroft, J.A. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 2001, 14, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.; Rowley, J.; Vander Hoorn, S.; Wijesooriya, N.S.; Unemo, M.; Low, N.; Stevens, G.; Gottlieb, S.; Kiarie, J.; Temmerman, M. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE 2015, 10, e0143304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cudmore, S.L.; Delgaty, K.L.; Hayward-McClelland, S.F.; Petrin, D.P.; Garber, G.E. Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis. Clin. Microbiol. Rev. 2004, 17, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Than, L.T.L. Vulvovaginal candidosis: Contemporary challenges and the future of prophylactic and therapeutic approaches. Mycoses 2016, 59, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A. Vulvovaginal Candida albicans infections: Pathogenesis, immunity and vaccine prospects. BJOG-Int. J. Obstet. Gynaecol. 2015, 122, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.N.; McClelland, R.S. Global epidemiology of Trichomonas vaginalis. Sex. Transm. Infect. 2013, 89, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, A.; Minu, M.; Wakode, S.; Agrawal, S.; Narasimhan, B. Indazole: A medicinally important heterocyclic moiety. Med. Chem. Res. 2012, 21, 1509–1523. [Google Scholar] [CrossRef]
- Lopez-Vallejo, F.; Castillo, R.; Yepez-Mulia, L.; Medina-Franco, J.L. Benzotriazoles and indazoles are scaffolds with biological activity against Entamoeba histolytica. J. Biomol. Screen. 2011, 16, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Marrero-Ponce, Y.; Meneses-Marcel, A.; Castillo-Garit, J.A.; Machado-Tugores, Y.; Escario, J.A.; Barrio, A.G.; Pereira, D.M.; Nogal-Ruiz, J.J.; Arán, V.J.; Martínez-Fernández, A.R.; et al. Predicting antitrichomonal activity: A computational screening using atom-based bilinear indices and experimental proofs. Bioorg. Med. Chem. 2006, 14, 6502–6524. [Google Scholar] [CrossRef] [PubMed]
- Minu, M.; Thangadurai, A.; Wakode, S.R.; Agrawal, S.S.; Narasimhan, B. Synthesis, antimicrobial activity and QSAR studies of new 2,3-disubstituted-3,3a,4,5,6,7-hexahydro-2H-indazoles. Bioorg. Med. Chem. Lett. 2009, 19, 2960–2964. [Google Scholar] [CrossRef] [PubMed]
- Tanitame, A.; Oyamada, Y.; Ofuji, K.; Kyoya, Y.; Suzuki, K.; Ito, H.; Kawasaki, M.; Nagai, K.; Wachi, M.; Yamagishi, J.-I. Design, synthesis and structure–activity relationship studies of novel indazole analogues as DNA gyrase inhibitors with Gram-positive antibacterial activity. Bioorg. Med. Chem. Lett. 2004, 14, 2857–2862. [Google Scholar] [CrossRef] [PubMed]
- Boehm, H.-J.; Boehringer, M.; Bur, D.; Gmuender, H.; Huber, W.; Klaus, W.; Kostrewa, D.; Kuehne, H.; Luebbers, T.; Meunier-Keller, N.; et al. Novel inhibitors of DNA gyrase: 3D structure based biased needle screening, hit validation by biophysical methods, and 3D guided optimization. A promising alternative to random screening. J. Med. Chem. 2000, 43, 2664–2674. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Lucio, O.; Naveja, J.J.; Vite-Caritino, H.; Prieto-Martínez, F.D.; Medina-Franco, J.L. Review. One drug for multiple targets: A computational perspective. J. Mex. Chem. Soc. 2016, 60, 168–181. [Google Scholar]
- Ni, H.; Wendoloski, J. Structure-based design of new antibacterial agents. In Annual Reports in Computational Chemistry; David, C.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 279–295. ISBN 978-0-444-52822-3. [Google Scholar]
- Pérez-Villanueva, J.; Medina-Franco, J.L.; Méndez-Lucio, O.; Yoo, J.; Soria-Arteche, O.; Izquierdo, T.; Lozada, M.C.; Castillo, R. CASE plots for the chemotype-based activity and selectivity analysis: A CASE study of cyclooxygenase inhibitors. Chem. Biol. Drug Des. 2012, 80, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Villanueva, J.; Méndez-Lucio, O.; Soria-Arteche, O.; Medina-Franco, J.L. Activity cliffs and activity cliff generators based on chemotype-related activity landscapes. Mol. Divers. 2015, 19, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar] [PubMed]
- Stenson, W.F.; Zhang, Z.; Riehl, T.; Stanley, S.L. Amebic infection in the human colon induces cyclooxygenase-2. Infect. Immun. 2001, 69, 3382–3388. [Google Scholar] [CrossRef] [PubMed]
- Rub, A.; Arish, M.; Husain, S.A.; Ahmed, N.; Akhter, Y. Host-lipidome as a potential target of protozoan parasites. Microbes Infect. 2013, 15, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Cadogan, J.I.G.; Mackie, R.K. 2-Phenylindazole. Org. Synth. 1968, 48, 113. [Google Scholar] [CrossRef]
- De Angelis, M.; Stossi, F.; Carlson, K.A.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. Indazole estrogens: Highly selective ligands for the estrogen receptor β. J. Med. Chem. 2005, 48, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Soria-Arteche, O.; Castillo, R.; Hernández-Campos, A.; Hurtado-de la Peña, M.; Navarrete-Vázquez, G.; Medina-Franco, J.L.; Gómez-Flores, K. Studies on the selective S-oxidation of albendazole, fenbendazole, triclabendazole, and other benzimidazole sulfides. J. Mex. Chem. Soc. 2005, 49, 353–358. [Google Scholar]
- Ohnmacht, S.A.; Culshaw, A.J.; Greaney, M.F. Direct arylations of 2H-indazoles on water. Org. Lett. 2010, 12, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Cedillo-Rivera, R.; Chávez, B.; González-Robles, A.; Tapia, A.; Yépez-Mulia, L. In vitro effect of nitazoxanide against Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis trophozoites. J. Eukaryot. Microbiol. 2002, 49, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Villanueva, J.; Hernández-Campos, A.; Yépez-Mulia, L.; Méndez-Cuesta, C.; Méndez-Lucio, O.; Hernández-Luis, F.; Castillo, R. Synthesis and antiprotozoal activity of novel 2-{[2-(1H-imidazol-1-yl)ethyl]sulfanyl}-1H-benzimidazole derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 4221–4224. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Document: M02-A12; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2015.
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Lill, A.; Scholich, K.; Stark, H. Synthesis of novel dansyl-labeled celecoxib derivatives. Tetrahedron Lett. 2013, 54, 6682–6686. [Google Scholar] [CrossRef]
- Orlando, B.J.; Malkowski, M.G. Crystal structure of rofecoxib bound to human cyclooxygenase-2. Acta Crystallogr. F 2016, 72, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Quintero, A.; Pelcastre, A.; Solano, J.D. Antitumoral activity of new pyrimidine derivatives of sesquiterpene lactones. J. Pharm. Pharm. Sci. 1999, 2, 108–112. [Google Scholar] [PubMed]
- Loza-Mejía, M.A.; Olvera-Vázquez, S.; Maldonado-Hernández, K.; Guadarrama-Salgado, T.; González-Sánchez, I.; Rodríguez-Hernández, F.; Solano, J.D.; Rodríguez-Sotres, R.; Lira-Rocha, A. Synthesis, cytotoxic activity, DNA topoisomerase-II inhibition, molecular modeling and structure-activity relationship of 9-anilinothiazolo[5,4-b]quinoline derivatives. Bioorg. Med. Chem. 2009, 17, 3266–3277. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.; Smith, D.M.; Shepherd, T.; Thompson, D. o-Nitrobenzylidene compounds. Part 3. Formation of 4-arylamino-3-methoxycinnoline 1-oxides from N-o-nitrobenzylideneanilines, cyanide ion, methanol: The intermediacy of 2-aryl-3-cyano-2H-indazole 1-oxides. J. Chem. Soc. Perk. Trans. 1987, 1, 495–500. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, C.; Larock, R.C.; Shi, F. Synthesis of 2H-indazoles by the [3 + 2] dipolar cycloaddition of sydnones with arynes. J. Org. Chem. 2011, 76, 8840–8851. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.R.; Park, A.; Park, N.; Lee, S. Consecutive condensation, C–N and N–N bond formations: A copper-catalyzed one-pot three-component synthesis of 2H-indazole. Org. Lett. 2011, 13, 3542–3545. [Google Scholar] [CrossRef] [PubMed]
- Cadogan, J.I.G.; Searle, R.J.G. Cyclizations induced by triethyl phosphite. Convenient new route to indazoles and triazoles. Chem. Ind. 1963, 1282–1283. [Google Scholar]
- Genung, N.E.; Wei, L.; Aspnes, G.E. Regioselective synthesis of 2H-indazoles using a mild, one-pot condensation–Cadogan reductive cyclization. Org. Lett. 2014, 16, 3114–3117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Sze, J.Y.-C.; Gross, J.L.; Robichaud, A.J. Preparation of Azacyclylbenzamide Derivatives as Histamine-3 Antagonists for Treating CNS Disorders. U.S. Patent 20080293771A1, 27 November 2008. [Google Scholar]
- Aspnes, G.E.; Didiuk, M.T.; Filipski, K.J.; Guzman-Perez, A.; Lee, E.C.Y.; Pfefferkorn, J.A.; Stevens, B.D.; Tu, M.M. Preparation of 3-[4-(Heterocyclyl-Substituted)-Benzamido]Propanoic Acids as Glucagon Receptor Modulators. U.S. Patent 20120202834A1, 9 August 2012. [Google Scholar]
- Lee, J.H.; Matsumoto, A.; Yoshida, M.; Simamura, O. New routes to 1,2-diazoles with a fused ring system by reductive and oxidative cyclizations. Chem. Lett. 1974, 3, 951–954. [Google Scholar] [CrossRef]
- Hattori, K.; Yamaguchi, K.; Yamaguchi, J.; Itami, K. Pd- and Cu-catalyzed C-H arylation of indazoles. Tetrahedron 2012, 68, 7605–7612. [Google Scholar] [CrossRef]
- Geng, X.; Wang, C. Rhenium-catalyzed [4 + 1] annulation of azobenzenes and aldehydes via isolable cyclic rhenium(I) complexes. Org. Lett. 2015, 17, 2434–2437. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Maestro, version 9.1; Schrödinger, LLC: New York, NY, USA, 2010.
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins Struct. Funct. Bioinform. 2009, 77, 114–122. [Google Scholar] [CrossRef] [PubMed]
- AutoDockTools, version 1.5.6; The Scripps Research Institute: La Jolla, CA, USA, 2013.
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |




| Compound | R1 | R2 | IC50 (µM) G. intestinalis | IC50 (µM) E. histolytica | IC50 (µM) T. vaginalis |
|---|---|---|---|---|---|
| 7 | H | – | 0.1133 ± 0.0218 | 0.0798 ± 0.0036 | 0.1184 ± 0.0218 |
| 8 | Cl | – | 0.0634 ± 0.0031 | 0.0415 ± 0.0031 | 0.1071 ± 0.0031 |
| 9 | OCH3 | – | 0.2051 ± 0.0063 | 0.1538 ± 0.0158 | 0.3723 ± 0.0158 |
| 10 | COOCH3 | – | 0.0634 ± 0.0056 | 0.0218 ± 0.0028 | 0.1070 ± 0.0056 |
| 11 | SCH3 | – | 0.2185 ± 0.0088 | 0.0978 ± 0.0147 | 0.2725 ± 0.0147 |
| 12 | OH | – | 0.1189 ± 0.0067 | 0.0737 ± 0.0101 | 0.1570 ± 0.0135 |
| 13 | COOH | – | 0.1931 ± 0.0119 | 0.0965 ± 0.0059 | 0.3274 ± 0.0178 |
| 14 | SOCH3 | – | 0.1678 ± 0.0110 | 0.0878 ± 0.0083 | 0.3121 ± 0.0110 |
| 15 | SO2CH3 | – | 0.0900 ± 0.0234 | 0.1359 ± 0.0052 | 0.1450 ± 0.0026 |
| 16 | H | H | 0.0518 ± 0.0052 | 0.3033 ± 0.0105 | 0.0573 ± 0.0026 |
| 17 | Cl | H | 0.0607 ± 0.0023 | 0.0213 ± 0.0023 | 0.1034 ± 0.0023 |
| 18 | COOCH3 | H | 0.0959 ± 0.0022 | 0.0502 ± 0.0022 | 0.1020 ± 0.0151 |
| 20 | COOH | H | 0.0795 ± 0.0045 | 0.0445 ± 0.0045 | 0.1113 ± 0.0180 |
| 21 | SO2CH3 | H | 0.1242 ± 0.0122 | 0.2081 ± 0.0061 | 0.2138 ± 0.0101 |
| 22 | H | Cl | 0.1132 ± 0.0070 | 0.0394 ± 0.0000 | 0.1181 ± 0.0046 |
| 23 | H | COOCH3 | 0.1188 ± 0.0086 | 0.0731 ± 0.0086 | 0.1431 ± 0.0043 |
| 25 | H | COOH | 0.1209 ± 0.0090 | 0.0509 ± 0.0000 | 0.2402 ± 0.0067 |
| 26 | H | SO2CH3 | 0.1062 ± 0.0081 | 0.0459 ± 0.0081 | 0.1837 ± 0.0162 |
| MTZ | – | – | 1.2260 ± 0.1250 | 0.3798 ± 0.1461 | 0.2360 ± 0.0160 |
| ABZ | – | – | 0.0370 ± 0.0030 | 56.5334 ± 18.8445 | 1.5905 ± 0.0113 |
| Compound | MIC (mM) C. albicans | MIC (mM) C. glabrata |
|---|---|---|
| 18 | 3.807 | 15.227 |
| 23 | 3.807 | 15.227 |
| Ketoconazole | 0.045 | 0.079 |
| Compound | Docking Score (Lowest Energy Conformation) | % of Inhibition of COX-2 |
|---|---|---|
| 7 1 | −8.0 | Inactive |
| 16 | −9.7 | Inactive |
| 18 | −9.5 | 50.01 ± 9.49 |
| 21 | −10.1 | 44.45 ± 2.65 |
| 23 | −10.0 | 36.35 ± 1.7 |
| 26 | −11.1 | 41.22 ± 5.93 |
| Celecoxib 2 | −11.7 | 64.92 ± 2.36 |
| Compound | % Viability (10 µM) HaCaT Cells 1 | % Viability (10 µM) HeLa Cells | IC50 (µM) HaCaT cells 2 | IC50 (µM) HeLa Cells |
|---|---|---|---|---|
| 16 | 95.01 ± 2.44 | 93.04 ± 4.57 | 93.65 ± 17.30 | 125.00 ± 29.60 |
| 18 | 96.25 ± 4.14 | 94.14 ± 3.31 | - | - |
| 23 | 97.83 ± 5.19 | 93.72 ± 7.48 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Villanueva, J.; Yépez-Mulia, L.; González-Sánchez, I.; Palacios-Espinosa, J.F.; Soria-Arteche, O.; Sainz-Espuñes, T.D.R.; Cerbón, M.A.; Rodríguez-Villar, K.; Rodríguez-Vicente, A.K.; Cortés-Gines, M.; et al. Synthesis and Biological Evaluation of 2H-Indazole Derivatives: Towards Antimicrobial and Anti-Inflammatory Dual Agents. Molecules 2017, 22, 1864. https://doi.org/10.3390/molecules22111864
Pérez-Villanueva J, Yépez-Mulia L, González-Sánchez I, Palacios-Espinosa JF, Soria-Arteche O, Sainz-Espuñes TDR, Cerbón MA, Rodríguez-Villar K, Rodríguez-Vicente AK, Cortés-Gines M, et al. Synthesis and Biological Evaluation of 2H-Indazole Derivatives: Towards Antimicrobial and Anti-Inflammatory Dual Agents. Molecules. 2017; 22(11):1864. https://doi.org/10.3390/molecules22111864
Chicago/Turabian StylePérez-Villanueva, Jaime, Lilián Yépez-Mulia, Ignacio González-Sánchez, Juan Francisco Palacios-Espinosa, Olivia Soria-Arteche, Teresita Del Rosario Sainz-Espuñes, Marco A. Cerbón, Karen Rodríguez-Villar, Ana Karina Rodríguez-Vicente, Miguel Cortés-Gines, and et al. 2017. "Synthesis and Biological Evaluation of 2H-Indazole Derivatives: Towards Antimicrobial and Anti-Inflammatory Dual Agents" Molecules 22, no. 11: 1864. https://doi.org/10.3390/molecules22111864
APA StylePérez-Villanueva, J., Yépez-Mulia, L., González-Sánchez, I., Palacios-Espinosa, J. F., Soria-Arteche, O., Sainz-Espuñes, T. D. R., Cerbón, M. A., Rodríguez-Villar, K., Rodríguez-Vicente, A. K., Cortés-Gines, M., Custodio-Galván, Z., & Estrada-Castro, D. B. (2017). Synthesis and Biological Evaluation of 2H-Indazole Derivatives: Towards Antimicrobial and Anti-Inflammatory Dual Agents. Molecules, 22(11), 1864. https://doi.org/10.3390/molecules22111864