Continuous Preparation of Hollow Polymeric Nanocapsules Using Self-Assembly and a Photo-Crosslinking Process of an Amphiphilic Block Copolymer
Abstract
:1. Introduction
2. Results and Discussions
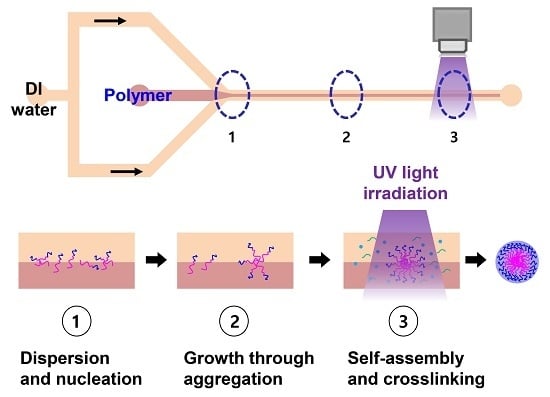
2.1. Continuous Preparation of HPNCs
2.2. The Mixing Time for Hydrodynamic Focusing Flow
2.3. Flow Visualization and Size Distribution of HPNCs
2.4. TEM, SEM, and Atomic Force Microscopy (AFM) Characterization of HPNCs
2.5. Measurement of the Refractive Index of HPNCs
2.6. Resistance Test of HPNCs to an Organic Solvent
3. Materials and Methods
3.1. Materials
3.2. Synthesis of SBR-b-PEGMA by Atom Transfer Radical Polymerization (ATRP)
3.3. Fabrication of Flow-Focusing Microfluidic Device
3.4. Characterization of HPNCs
3.5. Sample Preparation for the Measurement of the Refractive Index of HPNCs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bulović, V.; Khalfin, V.; Gu, G.; Burrows, P.; Garbuzov, D.; Forrest, S. Weak microcavity effects in organic light-emitting devices. Phys. Rev. B 1998, 58, 3730–3740. [Google Scholar] [CrossRef]
- Sun, Y.; Forrest, S.R. Enhanced light out-coupling of organic light-emitting devices using embedded low-index grids. Nat. Photon. 2008, 2, 483–487. [Google Scholar] [CrossRef]
- Kim, J.W.; Jang, J.H.; Oh, M.C.; Shin, J.W.; Cho, D.H.; Moon, J.H.; Lee, J.I. FDTD analysis of the light extraction efficiency of OLEDs with a random scattering layer. Opt. Express 2014, 22, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.C.; Park, J.H.; Jeon, H.J.; Go, J.S. Hollow-core polymeric nanoparticles for the enhancement of OLED outcoupling efficiency. Displays 2015, 37, 72–78. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Cho, S.-H.; Song, Y.-W.; Lee, Y.-J.; Lee, Y.-H.; Do, Y.R. Planarized SiNx/spin-on-glass photonic crystal organic light-emitting diodes. Appl. Phys. Lett. 2006, 89, 173502. [Google Scholar] [CrossRef]
- Jeon, S.; Kang, J.-W.; Park, H.-D.; Kim, J.-J.; Youn, J.R.; Shim, J.; Jeong, J.-H.; Choi, D.-G.; Kim, K.-D.; Altun, A.O. Ultraviolet nanoimprinted polymer nanostructure for organic light emitting diode application. Appl. Phys. Lett. 2008, 92, 223307. [Google Scholar] [CrossRef]
- Jang, J.-H.; Oh, M.-C. Outcoupling enhancement of OLEDs with a randomly distributed ito pattern fabricated by maskless wet etching method. J. Disp. Technol. 2013, 9, 900–903. [Google Scholar] [CrossRef]
- Koo, W.H.; Jeong, S.M.; Araoka, F.; Ishikawa, K.; Nishimura, S.; Toyooka, T.; Takezoe, H. Light extraction from organic light-emitting diodes enhanced by spontaneously formed buckles. Nat. Photon. 2010, 4, 222–226. [Google Scholar] [CrossRef]
- Chang, H.-W.; Lee, J.; Hofmann, S.; Hyun Kim, Y.; Müller-Meskamp, L.; Lüssem, B.; Wu, C.-C.; Leo, K.; Gather, M.C. Nano-particle based scattering layers for optical efficiency enhancement of organic light-emitting diodes and organic solar cells. J. Appl. Phys. 2013, 113, 204502. [Google Scholar] [CrossRef]
- Jang, W.D.; Yim, D.; Hwang, I.H. Photofunctional hollow nanocapsules for biomedical applications. J. Mater. Chem. B 2014, 2, 2202–2211. [Google Scholar] [CrossRef]
- Raichur, A.; Nakajima, Y.; Nagaoka, Y.; Maekawa, T.; Kumar, D.S. Hollow polymeric (PLGA) nano capsules synthesized using solvent emulsion evaporation method for enhanced drug encapsulation and release efficiency. Mater. Res. Express 2014, 1, 045407. [Google Scholar] [CrossRef]
- Son, K.J.; Yoon, H.J.; Kim, J.H.; Jang, W.D.; Lee, Y.; Koh, W.G. Photosensitizing hollow nanocapsules for combination cancer therapy. Angew. Chem. Int. Ed. Engl. 2011, 50, 11968–11971. [Google Scholar] [CrossRef] [PubMed]
- Tiarks, F.; Landfester, K.; Antonietti, M. Preparation of polymeric nanocapsules by miniemulsion polymerization. Langmuir 2001, 17, 908–918. [Google Scholar] [CrossRef]
- Jang, J.; Lee, K. Facile fabrication of hollow polystyrene nanocapsules by microemulsion polymerization. Chem. Commun. 2002, 1098–1099. [Google Scholar] [CrossRef]
- Khor, S.Y.; Truong, N.P.; Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Polymerization-induced self-assembly: The effect of end group and initiator concentration on morphology of nanoparticles prepared via RAFT aqueous emulsion polymerization. ACS Macro Lett. 2017, 6, 1013–1019. [Google Scholar] [CrossRef]
- Esser, L.; Truong, N.P.; Karagoz, B.; Moffat, B.A.; Boyer, C.; Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Gadolinium-functionalized nanoparticles for application as magnetic resonance imaging contrast agents via polymerization-induced self-assembly. Polym. Chem. 2016, 7, 7325–7337. [Google Scholar] [CrossRef]
- Truong, N.P.; Quinn, J.F.; Anastasaki, A.; Haddleton, D.M.; Whittaker, M.R.; Davis, T.P. Facile access to thermoresponsive filomicelles with tuneable cores. Chem. Commun. 2016, 52, 4497–4500. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Polymeric filomicelles and nanoworms: Two decades of synthesis and application. Polym. Chem. 2016, 7, 4295–4312. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Self-assembly of amphiphilic block copolymers in selective solvents. In Fluorescence Studies of Polymer Containing Systems; Springer: Gewerbestrasse, Switzerland, 2016; pp. 27–63. [Google Scholar]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Quemener, D.; Deratani, A.; Lecommandoux, S. Dynamic assembly of block-copolymers. Top. Curr. Chem. 2012, 322, 165–192. [Google Scholar] [PubMed]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Capretto, L.; Carugo, D.; Cheng, W.; Hill, M.; Zhang, X. Continuous-flow production of polymeric micelles in microreactors: Experimental and computational analysis. J. Colloid Interface Sci. 2011, 357, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Decker, C.; Nguyen Thi Viet, T. High-speed photocrosslinking of thermoplastic styrene–butadiene elastomers. J. Appl. Polym. Sci. 2000, 77, 1902–1912. [Google Scholar] [CrossRef]
- Capretto, L.; Cheng, W.; Carugo, D.; Katsamenis, O.L.; Hill, M.; Zhang, X. Mechanism of co-nanoprecipitation of organic actives and block copolymers in a microfluidic environment. Nanotechnology 2012, 23, 375602. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.T.; Jahn, A.; Locascio, L.E.; Vreeland, W.N. Controlled self-assembly of monodisperse niosomes by microfluidic hydrodynamic focusing. Langmuir 2010, 26, 8559–8566. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |














| Water (µL/min) | Polymer (µL/min) | Ratio R | τmix (s) |
|---|---|---|---|
| 80 | 40 | 0.5 | 0.03 |
| 80 | 60 | 0.75 | 0.05 |
| 80 | 80 | 1 | 0.07 |
| Wavelength (nm) | Photoresist AZ9260 | HPNCs + AZ9260 | ||
|---|---|---|---|---|
| Technical Data | Measured | Calculation | Measured | |
| 405 | 1.6862 | 1.6864 ± 0.0275 | 1.6846 | 1.6269 ± 0.0137 |
| 435 | 1.6722 | 1.6742 ± 0.0275 | 1.6707 | 1.6159 ± 0.0130 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, X.D.; Jeon, H.J.; Nguyen, V.T.; Park, D.H.; Lee, T.; Paik, H.-j.; Huh, J.; Go, J.S. Continuous Preparation of Hollow Polymeric Nanocapsules Using Self-Assembly and a Photo-Crosslinking Process of an Amphiphilic Block Copolymer. Molecules 2017, 22, 1892. https://doi.org/10.3390/molecules22111892
Nguyen XD, Jeon HJ, Nguyen VT, Park DH, Lee T, Paik H-j, Huh J, Go JS. Continuous Preparation of Hollow Polymeric Nanocapsules Using Self-Assembly and a Photo-Crosslinking Process of an Amphiphilic Block Copolymer. Molecules. 2017; 22(11):1892. https://doi.org/10.3390/molecules22111892
Chicago/Turabian StyleNguyen, Xuan Don, Hyeong Jin Jeon, Van Tien Nguyen, Dong Hyeok Park, Taeheon Lee, Hyun-jong Paik, June Huh, and Jeung Sang Go. 2017. "Continuous Preparation of Hollow Polymeric Nanocapsules Using Self-Assembly and a Photo-Crosslinking Process of an Amphiphilic Block Copolymer" Molecules 22, no. 11: 1892. https://doi.org/10.3390/molecules22111892
APA StyleNguyen, X. D., Jeon, H. J., Nguyen, V. T., Park, D. H., Lee, T., Paik, H. -j., Huh, J., & Go, J. S. (2017). Continuous Preparation of Hollow Polymeric Nanocapsules Using Self-Assembly and a Photo-Crosslinking Process of an Amphiphilic Block Copolymer. Molecules, 22(11), 1892. https://doi.org/10.3390/molecules22111892