Design, Synthesis and Preliminary Biological Evaluation of Novel Benzyl Sulfoxide 2-Indolinone Derivatives as Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
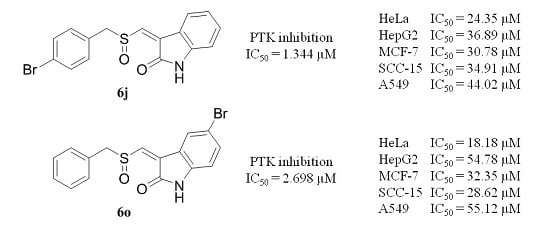
2.2. Biological Activity
3. Experimental Section
3.1. Chemistry
General Procedure for the Synthesis of Compounds (6a–6p)
3.2. Biological Evaluation
3.2.1. Tyrosine Kinase Inhibitory Activity Assay
3.2.2. Cytotoxic Activity Assays
Cell Culture
MTS Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer development, progression, and therapy: An epigenetic overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef] [PubMed]
- Dhermain, F. Radiotherapy of high-grade gliomas: Current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches. Chin. J. Cancer 2014, 33, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Rask-Andersen, M.; Masuram, S.; Schiöth, H.B. Evaluation of the targets for drugs in clinical trials suggests major shift in molecular class and indication. Annu. Rev. Pharm. Toxcol. 2014, 54, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Zwick, E.; Bange, J.; Ullrich, A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr. Relat. Cancer 2001, 8, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, P.L.; Gray, N.S. Targetting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 2009, 9, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, J.; Baselga, J. Epidermal growth factor receptor targeting in cancer. Semin. Oncol. 2006, 33, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Rask-Andersen, M.; Zhang, J.; Fabbro, D.; Schiöth, H.B. Advances in kinase targeting: Current clinical use and clinical trials. Trends Pharmacol. Sci. 2014, 34, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Hojjat-Farsangi, M. Small-Molecule Inhibitors of the Receptor Tyrosine Kinases: Promising Tools for Targeted Cancer Therapies. Int. J. Mol. Sci. 2014, 15, 13768–13801. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Nielsen, T.E.; Clausen, M.H. FDA-approved small-molecule kinase Inhibitors. Trends Pharmacol. Sci. 2015, 36, 422–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, P.A.; Murray, B.W. Protein kinase biochemistry and drug discovery. Bioorg. Chem. 2011, 39, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Hoessel, R.; Leclerc, S.; Endicott, J.A.; Nobel, M.E.; Lawrie, A.; Tunnah, P.; Leost, M.; Damiens, E.; Marie, D.; Marko, D.; et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell. Biol. 1999, 1, 60–67. [Google Scholar] [PubMed]
- Gribble, G.W. Heterocycle Scaffold II: Reactions and Applications of Indoles; Springer: Berlin, Germany, 2010; Volume 26. [Google Scholar]
- Pavlovska, T.L.; Redkin, R.G.; Lipson, V.V.; Atamanuk, D.V. Molecular diversity of spirooxindoles. Synthesis and biological activity. Mol. Divers. 2016, 20, 299–344. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.A.; Shawver, L.K.; Sun, L.; Tang, C.; App, H.; Powell, T.J.; Kim, Y.H.; Schreck, R.; Wang, X.; Risau, W.; et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999, 59, 99–106. [Google Scholar] [PubMed]
- O’Farrell, A.M.; Abrams, T.J.; Yuen, H.A.; Ngai, T.J.; Louie, S.G.; Yee, K.W.; Wong, L.M.; Hong, W.; Lee, L.B.; Town, A.; et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 2003, 101, 3597–3605. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Le, P.; Liang, C.; Chan, J.; Kiewlich, D.; Miller, T.; Harris, D.; Sun, L.; Rice, A.; Vasile, S.; et al. Potent and selective inhibitors of the Met [hepatocyte growth factor/scatter factor (HGF/SF) receptor] tyrosine kinase block HGF/SF-induced tumor cell growth and invasion. Mol. Cancer Ther. 2003, 2, 1085–1092. [Google Scholar] [PubMed]
- Laird, A.D.; Vajkoczy, P.; Shawver, L.K.; Thurnher, A.; Liang, C.; Mohammadi, M.; Schlessinger, J.; Ullrich, A.; Hubbard, S.R.; Blake, R.A.; et al. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer. Res. 2000, 60, 4152–4160. [Google Scholar] [PubMed]
- Chaudhary, N.I.; Roth, G.J.; Hilberg, F.; Müller-Quernheim, J.; Prasse, A.; Zissel, G.; Schnapp, A.; Park, J.E. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur. Respir. J. 2007, 29, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Smolen, G.A.; Sordella, R.; Muir, B.; Mohapatra, G.; Barmettler, A.; Archibald, H.; Kim, W.J.; Okimoto, R.A.; Bell, D.W.; Sgroi, D.C.; et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc. Natl. Acad. Sci. USA 2006, 103, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Mologni, L.; Rostagno, R.; Brussolo, S.; Knowles, P.P.; Kjaer, S.; Murray-Rust, J.; Rosso, E.; Zambon, A.; Scapozza, L.; McDonald, N.Q.; et al. Synthesis, structure-activity relationship and crystallographic studies of 3-substituted indolin-2-one RET inhibitors. Bioorg. Med. Chem. 2010, 18, 1482–1496. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Shaik, A.B.; Jain, N.; Kishor, C.; Nagabhushana, A.; Supriya, B.; Bharath Kumar, G.; Chourasiya, S.S.; Suresh, Y.; Mishra, R.K.; et al. Design and synthesis of pyrazole-oxindole conjugates targeting tubulin polymerization as new anticancer agents. Eur. J. Med. Chem. 2015, 92, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Nesi, G.; Sestito, S.; Mey, V.; Ricciardi, S.; Falasca, M.; Danesi, R.; Lapucci, A.; Breschi, M.C.; Fogli, S.; Rapposelli, S. Synthesis of Novel 3,5-Disubstituted-2-oxindole Derivatives As Antitumor Agents against Human Nonsmall Cell Lung Cancer. ACS Med. Chem. Lett. 2013, 4, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Li, C.; Zhan, Y.; Liu, J.; Luo, T.; Yan, H.; Zhang, S.; Li, W.; Wen, X.; et al. Substance Having Tyrosine Kinase Inhibitory Activity and Preparation Method and Use Thereof. U.S. Patent 2016151334A1, 2 June 2016. [Google Scholar]
- Praveen Kumar, S.; Gut, J.; Guedes, R.C.; Rosenthal, P.J.; Santos, M.M.; Moreira, R. Design, synthesis and evaluation of 3-methylene-substituted indolinones as antimalarials. Eur. J. Med. Chem. 2011, 46, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Zhdanko, A.G.; Gulevich, A.V.; Nenajdenko, V.G. One-step synthesis of N-acetylcysteine and glutathione derivatives using the Ugi reaction. Tetrahedron 2009, 65, 4692–4702. [Google Scholar] [CrossRef]
- Chahrour, O.; Abdalla, A.; Lam, F.; Midgley, C.; Wang, S. Synthesis and biological evaluation of benzyl styrylsulfonyl derivatives as potent anticancer mitotic inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 3066–3069. [Google Scholar] [CrossRef] [PubMed]
- Han, G.L.; Shang, N.Y.; Du, G.H. HTS model for protein tyrosine kinase inhibitors. Chin. Pharmacol. Bull. 2005, 21, 628–631. [Google Scholar]
- Munikrishnappa, C.S.; Puranik, S.B.; Kumar, G.V.; Prasad, Y.R. Part-1: Design, synthesis and biological evaluation of novel bromo-pyrimidine analogs as tyrosine kinase inhibitors. Eur. J. Med. Chem. 2016, 119, 70–82. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 6a–6p are available from the authors. |


| Compound | Tyrosine Kinase Inhibitory Activity |
|---|---|
| IC50 (μM) | |
| 6a | 3.16 ± 0.31 |
| 6b | 3.74 ± 0.19 |
| 6c | 9.32 ± 0.66 |
| 6d | 9.30 ± 0.47 |
| 6e | 5.09 ± 0.12 |
| 6f | 5.96 ± 0.14 |
| 6g | 3.30 ± 0.03 |
| 6h | 3.52 ± 0.15 |
| 6i | 3.32 ± 0.19 |
| 6j | 1.34 ± 0.03 |
| 6k | 2.23 ± 0.14 |
| 6l | 16.02 ± 0.05 |
| 6m | 3.85 ± 0.09 |
| 6n | 4.39 ± 0.23 |
| 6o | 2.69 ± 0.29 |
| 6p | 7.93 ± 0.20 |
| Indo 5 | 11.60 ± 0.79 |
| Compound | HeLa | MCF-7 | HepG2 | SCC-15 | A549 |
|---|---|---|---|---|---|
| IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | IC50 (μM) | |
| 6a | 11.17 ± 0.42 | 35.18 ± 1.16 | 52.08 ± 0.94 | 58.85 ± 0.75 | >100 |
| 6b | 13.99 ± 0.27 | 49.12 ± 1.08 | 47.82 ± 1.47 | >100 | >100 |
| 6c | 24.37 ± 0.54 | 31.54 ± 0.82 | 42.02 ± 0.70 | 49.87 ± 0.93 | >100 |
| 6d | 25.02 ± 0.68 | 49.12 ± 0.29 | 36.25 ± 0.53 | 64.97 ± 1.47 | 90.28 ± 2.14 |
| 6e | 70.88 ± 1.17 | >100 | 96.83 ± 0.95 | >100 | >100 |
| 6f | 60.27 ± 1.98 | 93.02 ± 1.50 | 86.50 ± 1.15 | 96.78 ± 3.76 | >100 |
| 6g | 40.92 ± 0.88 | 92.63 ± 0.97 | 75.35 ± 0.72 | >100 | >100 |
| 6h | 79.87 ± 1.57 | >100 | 98.29 ± 2.04 | >100 | >100 |
| 6i | 48.42 ± 0.14 | 75.06 ± 1.93 | 71.77 ± 2.18 | 82.14 ± 3.34 | 64.91 ± 1.57 |
| 6j | 24.05 ± 0.52 | 30.77 ± 0.29 | 36.90 ± 0.49 | 34.90 ± 0.34 | 44.01 ± 1.89 |
| 6k | 22.67 ± 0.64 | 59.08 ± 037 | 49.36 ± 1.33 | 86.72 ± 5.16 | 62.34 ± 2.00 |
| 6l | >100 | 81.57 ± 2.62 | >100 | >100 | >100 |
| 6m | 27.03 ± 1.20 | 41.43 ± 2.20 | 36.04 ± 2.13 | 50.33 ± 1.51 | 79.32 ± 0.79 |
| 6n | 27.76 ± 0.78 | 55.09 ± 1.51 | 58.99 ± 0.97 | 90.49 ± 2.32 | 59.56 ± 0.97 |
| 6o | 18.22 ± 0.66 | 32.34 ± 0.77 | 54.44 ± 0.88 | 28.63 ± 1.58 | 54.863 ± 0.66 |
| 6p | 19.83 ± 0.25 | 76.42 ± 1.50 | >100 | >100 | >100 |
| Indo 5 | 19.64 ± 0.46 | 24.19 ± 0.49 | 22.15 ± 0.41 | 28.16 ± 1.08 | 29.16 ± 0.29 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Peng, T.; Wang, G.; Wen, X.; Sun, Y.; Zhang, S.; Liu, S.; Wang, L. Design, Synthesis and Preliminary Biological Evaluation of Novel Benzyl Sulfoxide 2-Indolinone Derivatives as Anticancer Agents. Molecules 2017, 22, 1979. https://doi.org/10.3390/molecules22111979
Tang L, Peng T, Wang G, Wen X, Sun Y, Zhang S, Liu S, Wang L. Design, Synthesis and Preliminary Biological Evaluation of Novel Benzyl Sulfoxide 2-Indolinone Derivatives as Anticancer Agents. Molecules. 2017; 22(11):1979. https://doi.org/10.3390/molecules22111979
Chicago/Turabian StyleTang, Lin, Tao Peng, Gang Wang, Xiaoxue Wen, Yunbo Sun, Shouguo Zhang, Shuchen Liu, and Lin Wang. 2017. "Design, Synthesis and Preliminary Biological Evaluation of Novel Benzyl Sulfoxide 2-Indolinone Derivatives as Anticancer Agents" Molecules 22, no. 11: 1979. https://doi.org/10.3390/molecules22111979
APA StyleTang, L., Peng, T., Wang, G., Wen, X., Sun, Y., Zhang, S., Liu, S., & Wang, L. (2017). Design, Synthesis and Preliminary Biological Evaluation of Novel Benzyl Sulfoxide 2-Indolinone Derivatives as Anticancer Agents. Molecules, 22(11), 1979. https://doi.org/10.3390/molecules22111979