The Protective Effects of Astaxanthin on the OVA-Induced Asthma Mice Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Sensitization and Provocation of Airway Inflammation with OVA
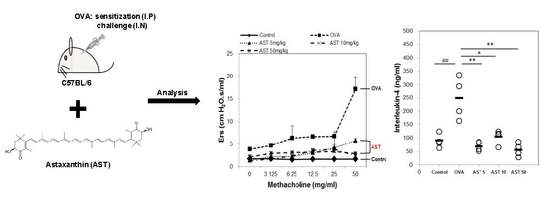
2.4. Assessment of Airway Hyperresponsiveness on the AST in OVA-Induced Asthmatic Mice
2.5. Measurement of Inflammatory Cytokine and Immunoglobulin Production in OVA or AST Exposed Mice
2.6. Histological Analysis of Lung Tissue in OVA or AST Exposed Mice
2.7. Statistical Analysis
3. Results
3.1. Effects of AST on Airway Hyperresponsiveness (AHR)
3.2. Effects of AST on Cytokines and BAL Total Cells in OVA-Induced Asthmatic Mice
3.3. Effects of AST on the Release of Immunoglobulins in Serum
3.4. Effects of AST on Histological Changes in Asthmatic Mice
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Braman, S.S. The global burden of asthma. Chest 2006, 130, 4S–12S. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, T.; Skadberg, B.T.; Eide, G.E.; Røksund, O.; Aksnes, L.; Øymar, K. Characteristics of asthma and airway hyper-responsiveness after premature birth. Pediatr. Allergy Immunol. 2005, 16, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Lee, M.Y.; Cho, E.S.; Choi, E.Y.; Son, H.Y.; Lee, K.Y. Effects of maternal exposure to di(2-ethylhexyl)phthalate (DEHP) during pregnancy on susceptibility to neonatal asthma. Toxicol. Appl. Pharmacol. 2014, 274, 402–407. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lv, L.; Wang, Z.; Huo, C.; Zheng, Z.; Yin, B.; Jiang, P.; Yang, Y.; Li, J.; Gao, Y.; et al. Pulvis Fellis Suis extract attenuates ovalbumin-induced airway inflammation in murine model of asthma. J. Ethnopharmacol. 2017, 207, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. The airway epithelium is central to the pathogenesis of asthma. Allergol. Int. 2008, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Hessel, E.M. Functions of T cells in asthma: More than just T(H)2 cells. Nat. Rev. Immunol. 2010, 10, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Bianco, A.; Catena, E.; De Palma, R.; Abbate, G.F. Th1/Th2 lymphocyte polarization in asthma. Allergy 2000, 55, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Nobuyoshi, S.; Masafumi, G.; Wataru, M. Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar]
- Meephansan, J.; Rungjang, A.; Yingmema, W.; Deenonpoe, R.; Ponnikorn, S. Effect of astaxanthin on cutaneous wound healing. Clin. Cosmet. Investig. Dermatol. 2017, 10, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Okada, S.; Toyokuni, S. Toyokuni, Astaxanthin ameliorates ferric nitrilotriacetate-induced renal oxidative injury in rats. J. Clin. Biochem. Nutr. 2017, 61, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, Z.; Cui, H.; Wang, Y.; Zhong, C. Astaxanthin protects astrocytes against trauma-induced apoptosis through inhibition of NKCC1 expression via the NF-κB signaling pathway. BMC Neurosci. 2017, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, A.; Dargahi, L.; Abbaszadeh, F.; Pourgholami, M.H.; Asgari, A.; Manoochehri, M.; Jorjani, M. Neuroprotective effects of astaxanthin in a rat model of spinal cord injury. Behav. Brain Res. 2017, 329, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-inflammatory effect of Astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp. Dermatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Cui, R.; Li, Z.; Liu, C.; Zhang, J. Astaxanthin alleviated acute lung injury by inhibiting oxidative/nitrative stress and the inflammatory response in mice. Biomed. Pharmacother. 2017, 95, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiong, H.; Cheng, Y.; Cui, C.; Zhang, X.; Xu, L.; Zhang, X. Effects of taraxasterol on ovalbumin-induced allergic asthma in mice. J. Ethnopharmacol. 2013, 148, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Paik, M.J.; Yee, S.T. Diisononyl phthalate induces asthma via modulation of Th1/Th2 equilibrium. Toxicol. Lett. 2017, 272, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ebmeier, S.; Thayabaran, D.; Braithwaite, I.; Bénamara, C.; Weatherall, M.; Beasley, R. Trends in international asthma mortality: Analysis of data from the WHO Mortality Database from 46 countries (1993–2012). Lancet 2017, 390, 935–945. [Google Scholar] [CrossRef]
- Bosnjak, B.; Stelzmueller, B.; Erb, K.J.; Epstein, M.M. Treatment of allergic asthma: Modulation of Th2 cells and their responses. Respir. Res. 2011, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhu, L. Update on the role of alternatively activated macrophages in asthma. J. Asthma Allergy 2016, 9, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol. Cells 2003, 16, 97–105. [Google Scholar] [PubMed]
- Mahmoud, F.F.; Haines, D.D.; Abul, H.T.; Abal, A.T.; Onadeko, B.O.; Wise, J.A. In vitro effects of astaxanthin combined with ginkgolide B on T lymphocyte activation in peripheral blood mononuclear cells from asthmatic subjects. J. Pharmacol. Sci. 2004, 94, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Kerstjens, H.A. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 158, S187–S192. [Google Scholar] [CrossRef] [PubMed]
- Brannan, J.D.; Lougheed, M.D. Airway hyperresponsiveness in asthma: Mechanisms, clinical significance, and treatment. Front. Physiol. 2012, 3, 460. [Google Scholar] [CrossRef] [PubMed]
- Steinke, J.W.; Borish, L. Th2 cytokines and asthma—Interleukin-4: Its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir. Res. 2001, 2, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.L.; Brown, M.A. Regulation of IL-4 production in mast cells: A paradigm for cell-type-specific gene expression. Immunol. Rev. 2001, 179, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Sehmi, R.; Nair, P. Anti-IL5 therapy for asthma and beyond. World Allergy Organ. J. 2014, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.K.; Fonseca, B.P.; Barboza, B.A.; Viola, J.P. The role of interferon-gamma on immune and allergic responses. Mem. Inst. Oswaldo Cruz 2005, 100 (Suppl. 1), 137–144. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Sullivan, B.M.; Stemmann, C.; Satoskar, A.R.; Sleckman, B.P.; Glimcher, L.H. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 2002, 295, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Snapper, C.M.; Paul, W.E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 1987, 236, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Saetta, M.; Turato, G. Airway pathology in asthma. Eur. Respir. J. Suppl. 2001, 34, 18s–23s. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Wynn, T.A. Pulmonary fibrosis: Pathogenesis, etiology and regulation. Mucosal Immunol. 2009, 2, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Masta, S.; Meyers, D.; Narayanan, A.S. Collagen synthesis by normal and fibrotic human lung fibroblasts and the effect of transforming growth factor-beta. Am. Rev. Respir. Dis. 1989, 140, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Madouri, F.; Guillou, N.; Fauconnier, L.; Marchiol, T.; Rouxel, N.; Chenuet, P.; Ledru, A.; Apetoh, L.; Ghiringhelli, F.; Chamaillard, M.; et al. Caspase-1 activation by NLRP3 inflammasome dampens IL-33-dependent house dust mite-induced allergic lung inflammation. J. Mol. Cell Biol. 2015, 7, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Iwata, A.; Nishio, K.; Winn, R.K.; Chi, E.Y.; Henderson, W.R., Jr.; Harlan, J.M. A broad-spectrum caspase inhibitor attenuates allergic airway inflammation in murine asthma model. J. Immunol. 2003, 170, 3386–3391. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.-H.; Hong, S.-G.; Mun, S.-K.; Kim, S.-J.; Lee, S.-J.; Kim, J.-J.; Kang, K.-Y.; Yee, S.-T. The Protective Effects of Astaxanthin on the OVA-Induced Asthma Mice Model. Molecules 2017, 22, 2019. https://doi.org/10.3390/molecules22112019
Hwang Y-H, Hong S-G, Mun S-K, Kim S-J, Lee S-J, Kim J-J, Kang K-Y, Yee S-T. The Protective Effects of Astaxanthin on the OVA-Induced Asthma Mice Model. Molecules. 2017; 22(11):2019. https://doi.org/10.3390/molecules22112019
Chicago/Turabian StyleHwang, Yun-Ho, Seong-Gyeol Hong, Seul-Ki Mun, Su-Jin Kim, Sung-Ju Lee, Jong-Jin Kim, Kyung-Yun Kang, and Sung-Tae Yee. 2017. "The Protective Effects of Astaxanthin on the OVA-Induced Asthma Mice Model" Molecules 22, no. 11: 2019. https://doi.org/10.3390/molecules22112019
APA StyleHwang, Y. -H., Hong, S. -G., Mun, S. -K., Kim, S. -J., Lee, S. -J., Kim, J. -J., Kang, K. -Y., & Yee, S. -T. (2017). The Protective Effects of Astaxanthin on the OVA-Induced Asthma Mice Model. Molecules, 22(11), 2019. https://doi.org/10.3390/molecules22112019