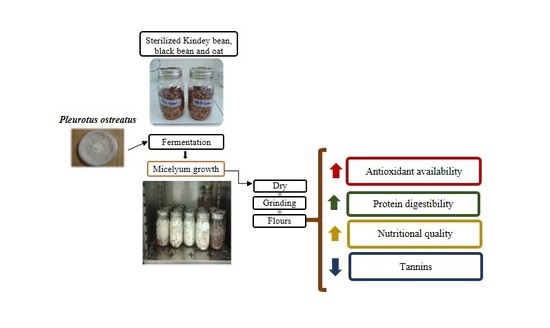
Increasing Antioxidant Activity and Protein Digestibility in Phaseolus vulgaris and Avena sativa by Fermentation with the Pleurotus ostreatus Fungus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximal Chemical Analysis
2.2. Antioxidant Activity and Total Phenols
2.3. In Vitro Digestibility, Soluble Nitrogen and Tannins
2.4. Amino Acid Profile
3. Materials and Methods
3.1. Seeds, Microorganism and Maintenance
3.2. Inoculum Production
3.3. Proximal Chemical Analysis
3.4. Simulated In Vitro Digestion
3.5. Antioxidant Activity
3.6. Total Phenol Content
3.7. Protein Digestibility
3.8. Soluble Nitrogen
3.9. Tannins Content
3.10. Amino Acids Profile
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Betoret, E.; Betoret, N.; Vidaland, D.; Fito, P. Functional foods development: Trends and technologies. Trends Food Sci. Technol. 2011, 22, 498–508. [Google Scholar] [CrossRef]
- Caballero, B.; Allen, L.; Prentice, A. Encyclopedia of Human Nutrition, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 366–371. ISBN 9780123848857. [Google Scholar]
- Sathe, S.K.; Deshpande, S.S. Encyclopedia of Food Science and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 403–412. ISBN 9780080917917. [Google Scholar]
- Herrera, F.; Betancur, D.; Segura, M.R. Compuestos bioactivos de la dieta con potencial en la prevención de patologías relacionadas con sobrepeso y obesidad; péptidos biológicamente activos. Nutr. Hosp. 2014, 29, 10–20. [Google Scholar]
- Xu, B.J.; Yuan, S.H.; Chang, S.K. Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. J. Food Sci. 2007, S167–S177. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.W.; Xie, W.H. Effect of different processing methods on certain antinutritional factors and protein digestibility in green and white faba bean (Vicia. faba L.). CyTA J. Food 2012, 11, 43–49. [Google Scholar] [CrossRef]
- Astiasarán, I.; Martínez, J.A. Alimentos Composición y Propiedades, 2nd ed.; McGraw Hill—Interamericana: Madrid, Spain, 2000; pp. 135–168. ISBN 84-486-0305-2. [Google Scholar]
- López, A.L.; Divo, D.; Pizzorno, M.; Villela, F.; Stella, A.M. Utilización de extractos de Avena sativa L. en dermatitis. Rev. Argent. Dermatol. 2006, 87, 100–105. [Google Scholar]
- Sharma, S.P.; Yadav, R.K.; Pokhrel, C.P. Growth and Yield of Oyster mushroom (Pleurotus. ostreatus) on different substrates. JNBR 2013, 2, 3–8. [Google Scholar]
- Sánchez, C. Cultivation of Pleurotus. ostreatus and other edible mushrooms. Appl. Microbiol. Biotechnol. 2010, 85, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, R.C.; Morris, Q.H.; Danoso, F.C.; Martínez, M.C.; Ramos, S.E. Influencia de la luz en la calidad proteica de Pleurotus ostreatus var. Florida. Rev. Cubana. Investig. Bioméd. 2003, 22, 226–231. [Google Scholar]
- Bautista, M.; Alanís, M.; González, E.; García, C. Composición química de tres cepas mexicanas de setas (Pleurotus ostreatus). Archiv. Latinoam. Nutr. 1998, 48, 359–363. [Google Scholar]
- Taofi, O.; Heleno, S.; Calhelha, R.; Alves, M.; Barros, L.; Barreiro, M.; González, A.; Ferreira, I. Development of Mushroom-Based Cosmeceutical Formulations with Anti-Inflammatory, Anti-Tyrosinase, Antioxidant, and Antibacterial Properties. Molecules 2016, 21, 1372. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Thomas, P.; Geraldine, P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus. ostreatus. Innov. Food Sci. Emerg. Technol. 2009, 10, 228–234. [Google Scholar] [CrossRef]
- Certík, M.; Sláviková, L.; Masrnová, S.; Sjbidor, J. Enhancement of Nutritional Value of Cereals with g-Linolenic Acid by Fungal Solid-State Fermentations. Food Technol. Biotechnol. 2006, 44, 75–82. [Google Scholar]
- Davila, M.A.; Sangronis, E.; Granito, M. Leguminosas germinadas o fermentadas: Alimentos o ingredientes de alimentos funcionales. Archiv. Latinoam. Nutr. 2003, 53, 348–354. [Google Scholar]
- USDA. Agricultural Research Service; National Nutrient Database for Standard Reference. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 17 October 2017).
- Vega, A.; Franco, H. Productividad y calidad de los cuerpos fructíferos de los hongos comestibles Pleurotus pulmonarius RN2 y P. djamor RN81 y RN82 cultivados sobre sustratos lignocelulósicos. Inf. Tecnol. 2012, 24, 69–78. [Google Scholar] [CrossRef]
- Raya, J.; Gutiérrez, G.; Ramírez, J.; Prieto, J.; Aguirre, C. Caracterización de proteínas y contenido mineral de dos variedades nativas de frijol de México. Agron. Mesoam. 2014, 25, 1–11. [Google Scholar] [CrossRef]
- Deshpande, S.; Sathe, S.; Salunkhe, D. Interrelationships between certain physical and chemical properties of dry bean (Phaseolus vulgaris L.). Qual. Plant. Plant. Foods Hum. Nutr. 1984, 34, 53–65. [Google Scholar] [CrossRef]
- Morales, M.; Peña, C.; García, A.; Aguilar, G.; Kohashi, J. Características físicas y de germinación en semillas y plántulas de frijol (Phaseolus. Vulgaris L.) silvestre, domesticado y su progenie. Agrociencia 2017, 51, 43–62. [Google Scholar]
- Papaspyridi, L.; Aligiannis, N.; Topakas, E.; Christakopoulos, P.; Skaltsounis, A.; Fokialakis, N. Submerged Fermentation of the Edible Mushroom Pleurotus ostreatus in a Batch Stirred Tank Bioreactor as a Promising Alternative for the Effective Production of Bioactive Metabolites. Molecules 2012, 17, 2714–2724. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sakoda, A.; Suzuki, M. Biological efficiency and nutritional value of Pleurotus. ostreatus cultived on spent beer grain. Bioresour. Technol. 2001, 78, 293–300. [Google Scholar] [CrossRef]
- Zieliński, H.; Kozłowska, H. Antioxidant Activity and Total Phenolics in Selected Cereal Grains and Their Different Morphological Fractions. J. Agric. Food Chem. 2000, 48, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Vergara, H.; Gandul, B.; Roca, M. Formation of oxidised chlorophyll catabolites in olives. J. Food Comp. Anal. 2011, 24, 851–857. [Google Scholar] [CrossRef]
- Granito, M.; Paolini, M.; Pérez, S. Polyphenols and antioxidant capacity of Phaseolus. vulgaris extreme conditions and processed. LWT Food Sci. Technol. 2008, 41, 994–999. [Google Scholar] [CrossRef]
- Giardina, P.; Palmieri, G.; Fontanella, B.; Rivieccio, V.; Sannia, G. Manganese Peroxidase Isoenzymes Produced by Pleurotus. ostreatus Grown on Wood Sawdust. Arch. Biochem. Bioph. 2000, 376, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Cardador, M.A.; Loarca, P.G.; Dave, O.B. Antioxidant Activity in Common Beans (Phaseolus. vulgaris). J. Agric. Food Chem. 2002, 50, 6975–6980. [Google Scholar] [CrossRef]
- Peterson, M. Oats Antioxidants. J. Cereal Sci. 2001, 33, 115–129. [Google Scholar] [CrossRef]
- Labaneiah, M.; Luh, B. Changes of starch, crude fiber, and oligosaccharides in germinating dry beans. Cereal Chem. 1981, 58, 135–138. [Google Scholar]
- Sharma, R.; Aora, D. Fungal degradation of lignocellulosic residues: An aspect of improved nutritive quality. Crit. Rev. Microbiol. 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Chang, S.K. Total phenolic ceontent and antioxidant properties of eclipse black beans (Phaseolus. vulgaris L.) as affected by processing methods. J. Food Sci. 2008, 73, H19–H27. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Hoffmann, L.; Bhon, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Chen, K.; González, E. Impact of Commercial Precooking of Common Bean (Phaseolus. vulgaris) on the Generation of Peptides, After Pepsin–Pancreatin Hydrolysis, Capable to Inhibit Dipeptidyl Peptidase-IV. J. Food Sci. 2014, 80, H188–H198. [Google Scholar] [CrossRef] [PubMed]
- Ribero, D.; Patto, C.M.; Pinto, M. In vitro protein digestibility of enzymatically pre-treated bean (Phaseolus. vulgaris L.) flour using commercial protease and Bacillus sp. protease. Food Sci. Technol. 2010, 30, 94–99. [Google Scholar]
- Starzynska-Janiszewska, A.; Stodolak, B.; Mickowska, B. Effect of controlled lactic acid fermentation on selected bioactive and nutritional parameters of tempeh obtained from unhulled common bean (Phaseolus. vulgaris) seeds. J. Sci. Food Agric. 2014, 94, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Mkandawire, N.L.; Weier, S.A.; Weller, C.L.; Jackson, D.S.; Rose, D.J. Composition, in vitro digestibility, and sensory evaluation of extruded whole grain sorghum breakfast cereals. LWT Food Sci. Technol. 2015, 62, 662–667. [Google Scholar] [CrossRef]
- Tripathi, J.P.; Yadav, J.S. Optimisation of solid substrate fermentation of wheat straw into animal feed by Pleurotus. ostreatus: A pilot effort. Anim. Feed Sci. Technol. 2015, 662–667. [Google Scholar] [CrossRef]
- Aw, T.L.; Swanson, B.G. Influence of Tannin on Phaseolus. vulgaris Protein Digestibility and QualityAuthorsT-L. J. Food Sci. 1985, 50, 67–71. [Google Scholar] [CrossRef]
- Fan, L.; Pandey, A.; Mohan, R.; Soccol, C.R. Use of Various Coffee Industry Residues for the Cultivation of Pleurotus. ostreatus in Solid State Fermentation. Eng. Life Sci. 2000, 20, 41–52. [Google Scholar]
- Rodrigues, J.M.; Albino, S.; Pereira, D.; Dias, M.; Soares, J.; Cuquetto, H.; Megumi, M.C. Production of edible mushroom and degradation of antinutritional factors in jatropha biodiesel residues. Food Sci. Technol. 2013, 50, 575–580. [Google Scholar]
- Díaz, A.M.; Caldas, G.V.; Blair, M.W. Concentrations of condensed tannins and anthocyanins in common bean seed coats. Food Res. Int. 2010, 43, 595–601. [Google Scholar] [CrossRef]
- Martínez, D.A.; Buglione, M.B.; Filippi, M.V.; Reynoso, L.D.; Rodríguez, G.E.; Agüero, M.S. Evaluación del crecimiento micelial de Pleurotus ostreatus y Agrocybe aegerita sobre orujos de pera. An. Biol. 2005, 37, 1–10. [Google Scholar] [CrossRef]
- Badui, D.S. Quimica de los Alimentos, 4th ed.; Pearson Educación: Ciudad de México, Mexico, 2006; pp. 121–130. ISBN 970-26-0670-5. [Google Scholar]
- Hernández, C.; Gutiérrez, G.; Salcedo, S. Screening for decolorizing basidiomycetes in Mexico. Screening and Selectión of Ligninolytic basidiomycetes with decolorizing ability in Northeast Mexico. World J. Microbiol. Biotechnol. 2008, 24, 465–473. [Google Scholar] [CrossRef]
- Gan, R.; Li, H.; Gunaratne, A.; Sui, Z.; Corke, H. Effects of fermented edible seeds and their products on human health: Bioactive components and bioactivities. Comp. Rev. Food Sci. Food Saf. 2017, 16, 489–531. [Google Scholar] [CrossRef]
- Hu, J.; Duvnjak, Z. The production of a laccase and the decrease of the phenolic content in canola meal during the growth of the fungus Pleurotus. ostreatus in solid state fermentation processes. Eng. Life Sci. 2004, 4, 50–55. [Google Scholar] [CrossRef]
- Association of Analytical Communities (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; AOAC: Gaithersburg, MD, USA, 2006; ISBN 0935584773-9780935584776. [Google Scholar]
- Lamothe, S.; Corbeil, M.M.; Turgeon, S.L.; Britten, M. Influence of cheese matrix on lipid digestion in a simulated gastro-intestinal environment. Food Funct. 2012, 3, 681–774. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Aruguman, S.; Perumal, S. Effect of indigenous processing methods on phenolics and antioxidant potential of Underutilized legumes Acacia auriculiformis and Parkia. roxburghii. J. Food Qual. 2012, 36, 99–112. [Google Scholar] [CrossRef]
- Reyes, C.; Cuevas, E.; Milán, J.; Cárdenas, O.; Barrón, J. Solid state fermentation process for producing chickpea (Cicer arietinum L.) tempeh flour. Physicochemical and nutritional characteristics of the product. J. Sci. Food Agric. 2014, 84, 271–278. [Google Scholar] [CrossRef]
- Blanco, A. Importance of some factors on digestibility of black beans (Phaseolus. vulgaris) and of its amino acids in humans adults. Guatemala USAC/INCAP 1983, xi, 134. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Parameter (%) | BB | FBB | KB | FKB | OG | FOG |
|---|---|---|---|---|---|---|
| Protein | 23.62 ± 1.12 c | 22.80 ± 2.85 c | 22.81 ± 1.3 c | 25.78 ± 2.35 d | 11.78 ± 1.28 a | 12.56 ± 0.63 b |
| Fat | 2.08 ± 0.32 bc | 1.67 ± 0.34 abc | 1.86 ± 0.66 ab | 1.68 ± 0.17 a | 2.44 ± 0.45 c | 4.80 ± 0.60 d |
| Minerals | 4.90 ± 0.15 c | 4.58 ± 0.31 c | 4.63 ± 0.24 c | 4.02 ± 0.01 c | 1.61 ± 0.09 b | 0.83 ± 0.52 a |
| Fiber | 48.73 ± 0.56 f | 20.0 ± 0.51 c | 29.18 ± 0.15 d | 33.88 ± 0.28 e | 14.00 ± 0.07 b | 10.92 ± 0.70 a |
| Carbohydrates | 20.69 ± 0.79 a | 50.95 ± 0.96 d | 41.52 ± 0.59 c | 34.64 ± 1.5 b | 70.17 ± 0.44 e | 70.89 ± 0.66 f |
| FOLIN (mg Acid Gallic/g Flour) | DPPH (mg Trolox/g Flour) | |||||
|---|---|---|---|---|---|---|
| Flour | Initial | G.D. * | I.D. ** | Initial | G.D. | I.D. |
| BB | 1.48 ± 0.01 b | 1.94 ± 0.05 c | 3.30 ± 0.06 a | 1.24 ± 0.04 b | 2.89± 0.53 b | 8.97 ± 0.73 d |
| FBB | 1.87 ± 0.24 b | 2.10 ± 0.00 d | 6.85 ± 0.03 c | 1.73 ± 0.09 c | 3.90± 2.21 d | 13.31 ± 1.63 f |
| KB | 1.59 ± 0.10 b | 1.82 ± 0.02 ab | 4.23 ± 0.09 a | 1.2 ± 0.01 b | 2.42 ± 0.51 a | 7.2 ± 0.29 c |
| FKB | 1.59 ± 0.01 b | 1.77 ± 0.06 a | 4.78 ± 0.20 b | 1.2 ± 0.08 b | 3.24 ± 0.04 c | 9.39 ± 0.01e |
| OG | 0.85 ± 0.76 a | 1.88 ± 0.02 b | 2.72 ± 0.49 a | 0.40 ± 0.03 a | 2.27 ± 0.77 a | 3.04 ± 0.31 a |
| FOG | 2.89 ± 0.34 c | 2.12 ± 0.08 d | 4.91 ± 0.06 b | 1.30 ± 0.08 b | 2.85 ± 0.37 b | 5.04 ± 0.25 b |
| Flour | Protein Digestibility (%) | Soluble Nitrogen (%) | Tannin Content (mg/100 g) |
|---|---|---|---|
| BB | 39.99 ± 1.71 a | 0.60 ± 0.80 b | 65.21 ± 0.027 f |
| FBB | 48.13 ± 0.78 c | 1.34 ± 2.3 d | 22.07 ± 0.016 a |
| KB | 44.06 ± 1.71 b | 0.30 ± 1.0 a | 35.54 ± 0.086 d |
| FKB | 69.01 ± 1.14 de | 0.60 ± 1.5 b | 23.37 ± 0.017 b |
| OG | 63.25 ± 1.65 d | 0.61 ± 0.4 b | 55.67 ± 0.057 e |
| FOG | 70.01 ± 0.30 e | 0.91 ± 0.22 c | 28.11 ± 0.030 c |
| Flours | ||||||
|---|---|---|---|---|---|---|
| Amino Acid (mg/g Protein) | BB | FBB | KB | FKB | OG | FOG |
| Asparagine | 125.29 e | 124.22 c | 125.40 e | 124.88 d | 87.30 a | 88.33 b |
| Threonine | 46.11 c | 47.30 d | 49.51 e | 49.56 e | 37.04 a | 39.17 b |
| Serine | 53.10 d | 51.60 c | 53.68 e | 52.96 d | 46.74 b | 45.00 a |
| Glutamine | 157.89 c | 154.80 b | 153.63 a | 154.52 b | 217.81 e | 212.50 d |
| Proline | 46.58 b | 48.73 c | 34.71 a | 49.08 d | 60.85 e | 61.67 f |
| Glycine | 41.92 a | 43.00 b | 44.89 c | 43.25 b | 54.67 d | 56.67 e |
| Alanine | 42.85 a | 45.87 d | 44.89 b | 45.19 c | 50.26 e | 53.33 f |
| Valine | 59.62 b | 64.50 f | 60.62 c | 63.65 e | 57.32 a | 61.67 d |
| Methionine + Cysteine | 20.44 a | 22.45 c | 21.29 a | 21.87 b | 47.62 d | 49.19 d |
| Isoleucine | 49.84 c | 53.03 d | 49.98 c | 52.96 d | 41.45 a | 45.83 b |
| Leucine | 87.10 d | 88.87 e | 86.07 c | 89.89 f | 82.01 a | 84.17 b |
| Tyrosine | 30.74 c | 32.97 e | 32.39 d | 33.04 e | 27.34 a | 28.33 b |
| Phenylalanine | 61.95 d | 63.07 e | 60.62 c | 63.65 f | 56.44 a | 57.50 b |
| Hydroxylysine | 1.40 a | 3.34 d | 1.39 a | 1.94 b | 2.65 c | 3.33 d |
| Ornithine | 0.47 a | 0.96 d | 0.93 c | 0.97 d | 0.88 b | 1.67 e |
| Lysine | 68.93 d | 52.56 b | 68.49 d | 56.37 c | 47.62 b | 34.17 a |
| Histidine | 28.88 f | 25.80 d | 28.69 e | 25.27 c | 22.93 b | 20.83 a |
| Arginine | 57.29 c | 49.21 a | 60.62 d | 51.02 b | 67.02 e | 60.83 d |
| Tryptophan | 10.71 c | 10.51 b | 11.11 d | 11.18 d | 14.99 e | 10.00 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Páez, E.; Alanis-Guzmán, M.G.; Hernández-Luna, C.E.; Báez-González, J.G.; Amaya-Guerra, C.A.; Andrés-Grau, A.M. Increasing Antioxidant Activity and Protein Digestibility in Phaseolus vulgaris and Avena sativa by Fermentation with the Pleurotus ostreatus Fungus. Molecules 2017, 22, 2275. https://doi.org/10.3390/molecules22122275
Espinosa-Páez E, Alanis-Guzmán MG, Hernández-Luna CE, Báez-González JG, Amaya-Guerra CA, Andrés-Grau AM. Increasing Antioxidant Activity and Protein Digestibility in Phaseolus vulgaris and Avena sativa by Fermentation with the Pleurotus ostreatus Fungus. Molecules. 2017; 22(12):2275. https://doi.org/10.3390/molecules22122275
Chicago/Turabian StyleEspinosa-Páez, Edith, Ma. Guadalupe Alanis-Guzmán, Carlos E. Hernández-Luna, Juan G. Báez-González, Carlos A. Amaya-Guerra, and Ana M. Andrés-Grau. 2017. "Increasing Antioxidant Activity and Protein Digestibility in Phaseolus vulgaris and Avena sativa by Fermentation with the Pleurotus ostreatus Fungus" Molecules 22, no. 12: 2275. https://doi.org/10.3390/molecules22122275
APA StyleEspinosa-Páez, E., Alanis-Guzmán, M. G., Hernández-Luna, C. E., Báez-González, J. G., Amaya-Guerra, C. A., & Andrés-Grau, A. M. (2017). Increasing Antioxidant Activity and Protein Digestibility in Phaseolus vulgaris and Avena sativa by Fermentation with the Pleurotus ostreatus Fungus. Molecules, 22(12), 2275. https://doi.org/10.3390/molecules22122275