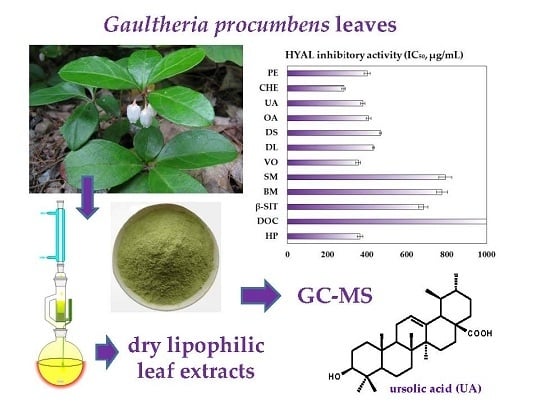
Metabolite Profiling of Eastern Teaberry (Gaultheria procumbens L.) Lipophilic Leaf Extracts with Hyaluronidase and Lipoxygenase Inhibitory Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative Profiling of G. procumbens Dry Lipophilic Leaf Extracts
2.2. Activity of G. procumbens Dry Lipophilic Leaf Extracts on Two Enzymes Involved in Inflammation
2.3. Seasonal Variation in the Content of Triterpene Acids in G. procumbens Leaves
3. Materials and Methods
3.1. General Information
3.2. Plant Material and Preparation of Plant Extracts
3.2.1. Plant Material
3.2.2. Preparation of Dry Lipophilic Leaf Extracts
3.2.3. Preparation of Plant Extracts for UHPLC-PDA Quantification of Triterpene Acids
3.3. Phytochemical Profiling
3.3.1. Qualitative GC-MS Analysis of G. procumbens Dry Leaf Extracts
3.3.2. Isolation and Structure Elucidation
3.3.3. Quantitative UHPLC-PDA Analysis of Triterpene Acids in G. procumbens Leaves
3.4. Biological Activity Testing
3.4.1. Hyaluronidase Inhibition Test
3.4.2. Lipoxygenase Inhibition Test
3.5. Statistical and Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, W.R.; Qiao, W.L.; Liu, Z.Z.; Wang, X.H.; Jiang, R.; Li, S.Y.; Shi, R.B.; She, G.M. Gaultheria: Phytochemical and pharmacological characteristics. Molecules 2013, 18, 12071–12108. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Marković, T.; Mojović, M.; Pejin, B.; Savić, A.; Perić, T.; Marković, D.; Stević, T.; Soković, M. Chemical composition and biological activity of Gaultheria procumbens L. essential oil. Ind. Crop. Prod. 2013, 49, 561–567. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Poulev, A.; Raskin, I. The determination of salicylates in Gaultheria procumbens for use as a natural aspirin alternative. J. Nutraceut. Function. Med. Foods 2003, 4, 39–52. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, R.; Sun, L.; Huang, C.; Wang, C.; Zhang, D.M.; Zhang, T.T.; Du, G.H. Anti-inflammatory activity of methyl salicylate glycosides isolated from Gaultheria yunnanensis (Franch.) Rehder. Molecules 2011, 16, 3875–3884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, L.; Liu, R.; Zhang, D.; Lan, X.; Huang, C.; Xin, W.; Wang, C.; Zhang, D.; Du, G. A novel naturally occurring salicylic acid analogue acts as an anti-inflammatory agent by inhibiting nuclear factor-kappaB activity in RAW264.7 macrophages. Mol. Pharm. 2012, 9, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Huang, C.; Zhang, X.; Zhang, G.; Ma, X.; Sun, L.; Wang, C.; Zhang, D.; Zhang, T.; Du, G. Evaluation of the new anti-inflammatory compound ethyl salicylate 2-O-β-d-glucoside and its possible mechanism of action. Intern. Immunopharmacol. 2013, 15, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.; Dobrowolska, A.; Kicel, A.; Owczarek, A.; Bazylko, A.; Granica, S.; Piwowarski, J.S.; Olszewska, M.A. Polyphenolic profile, antioxidant and anti-inflammatory activity of Eastern Teaberry (Gaultheria procumbens L.) leaf extracts. Molecules 2014, 19, 20498–20520. [Google Scholar] [CrossRef] [PubMed]
- Lytovchenko, A.; Beleggia, R.; Schauer, N.; Isaacson, T.; Leuendorf, J.E.; Hellmann, H.; Rose, J.K.; Fernie, A.R. Application of GC-MS for the detection of lipophilic compounds in diverse plant tissues. Plant Methods 2009, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lin, R.J.; Huang, J.C.; Wu, Y.H.; Cheng, M.J.; Lo, W.L. Chemical constituents from the whole plant of Gaultheria itoana Hayata. Chem. Biodivers. 2009, 6, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, D.; Li, C.; Zheng, J.; Koike, K.; Jia, Z.; Nikaido, T. Two diterpenoides from the roots of Gaultheria yunnanensis. J. Nat. Prod. 1999, 62, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Lu, Y.Y.; Su, X.J.; Huang, C.P.; Lu, X.W. A new dilactone from the seeds of Gaultheria yunnanesis. Fitoterapia 2010, 81, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.J.; Du, C.F.; Zheng, J.H.; Chen, X.Z. Studies of chemical constituents of Gaultheria leucocarpa var. yunnanensis. Zhongguo Zhonoyao Zazhi (Chin. J. Chin. Mat. Med.) 2001, 26, 844–846. [Google Scholar]
- Yang, M.F.; Li, Y.Y.; Li, B.G.; Zhang, G.L. A novel alkaloide from Gaultheria nummularioides. J. Asian Nat. Prod. Res. 2007, 9, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Eglinton, G.; Hamilton, R.J.; Martin-Smith, M. The alkane constituents of some New Zealand plants and their possible taxonomic implications. Phytochemistry 1962, 1, 137–145. [Google Scholar] [CrossRef]
- Córdova, C.; Gutiérrez, B.; Martínez-García, C.; Martín, R.; Gallego-Muñoz, P.; Hernández, M.; Nieto, M.L. Oleanolic acid controls allergic and inflammatory responses in experimental allergic conjunctivitis. PLoS ONE 2014, 9, e91282. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Yang, E.J.; Song, K.S.; Bae, J.S. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation 2013, 36, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, L.H.; Palazon, J.; Navarro-Ocaña, A. The pentacyclic triterpenes—Amyrins: A review of sources and biological activities. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; In Tech: Rijeka, Croatia, 2012. [Google Scholar]
- Loizou, S.; Lekakis, I.; Chrousos, G.P.; Moutsatsou, P. β-Sitosterol exhibits anti-inflammatory activity in human aortic endothelial cell. Mol. Nutr. Food Res. 2010, 54, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.; Owczarek, A.; Kosno, M.; Gontarek, D.; Matczak, M.; Olszewska, M.A. Variation in polyphenolic profile and in vitro antioxidant activity of eastern teaberry (Gaultheria procumbens L.) leaves following foliar development. Phytochem. Lett. 2017, in press. [Google Scholar] [CrossRef]
- Soni, U.; Brar, S.; Gauttam, V.K. Effect of seasonal variation on secondary metabolites of medicinal plants. IJPSR 2015, 6, 3654–3662. [Google Scholar]
- Markham, K.R.; Geiger, H. 1H Nuclear Magnetic Resonance spectroscopy of flavonoids and their glycosides in hexadeuterodimethylsulfoxide. In The Flavonoids. Advances in Research Since 1986; Harborne, J.B., Ed.; Chapman & Hall: Cambridge, UK, 1994; pp. 441–498. [Google Scholar]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1970. [Google Scholar]
- Li, Y.L.; Wu, L.; Ouyang, D.W.; Yu, P.; Xia, J.H.; Pan, Y.X.; Yang, X.W.; Zeng, H.W.; Cheng, X.R.; Jin, H.Z.; et al. Phenolic compounds of Abies nephrolepis and their NO production inhibitory activities. Chem. Biodivers. 2011, 8, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Wollenweber, E.; Kohorst, G. Novel epicuticular leaf flavonoids from Kalmia and Gaultheria (Ericaceae). Z. Naturforsch. 1984, 39C, 710–713. [Google Scholar]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B. Synergy-directed fractionation of botanical medicines: A case study with Goldenseal (Hydrastis canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Banfi, D.; Patiny, L. www.nmrdb.org: Resurrecting and processing NMR spectra on-line. Chimia 2008, 62, 280–281. [Google Scholar] [CrossRef]
- Yamano, Y.; Ito, M. Synthesis of optically active vomifoliol and roseoside stereoisomers. Chem. Pharm. Bull. 2005, 53, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Dalla Serra, A.; Franco, M.A.; Mattivi, F.; Ramponi, M.; Vacca, V.; Versini, G. Aroma characterization of Sardinian strawberry tree (Arbutus unedo L.) honey. Ital. J. Food Sci. 1999, 11, 47–56. [Google Scholar]
- Amakura, Y.; Yoshimura, M.; Sugimoto, N.; Yamazaki, T.; Yoshida, T. Marker constituents of the natural antioxidant Eucalyptus leaf extract for the evaluation of food additives. Biosci. Biotechnol. Biochem. 2009, 73, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, 140–146. [Google Scholar] [CrossRef]
- Souza, M.T.; Almeida, J.R.; Araujo, A.A.; Duarte, M.C.; Gelain, D.P.; Moreira, J.C.; dos Santos, M.R.; Quintas-Júnior, L.J. Structure-activity relationship of terpenes with anti-inflammatory profile—A systematic review. Basic Clin. Pharmacol. Toxicol. 2014, 115, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Bakovic, M. Biologically active triterpenoids and their cardioprotective and anti-inflammatory effects. J. Bioanal. Biomed. 2015, S12, 005. [Google Scholar]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Molecules 2010, 2, 2428–2466. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, L.; Skąpska, S.; Marszałek, K. Ursolic acid—A pentacyclic terpenoid with a wide spectrum of pharmacological activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [PubMed]
- Ogbe, R.J.; Ochalefu, D.O.; Mafulul, G.; Olaniru, O.B. A review on dietary phytosterols: Their occurrence, metabolism and health benefits. Asian J. Plant Sci. Res. 2015, 5, 10–21. [Google Scholar]
- Chua, T.; Eise, N.T.; Simpson, J.S.; Ventura, S. Pharmacological characterization and chemical fractionation of a liposterolic extract of saw palmetto (Serenoa repens): Effects on rat prostate contractility. J. Ethnopharmacol. 2014, 152, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.M.P. Review of Cucurbita pepo (pumpkin) its phytochemistry and pharmacology. Med. Chem. 2016, 6, 12–21. [Google Scholar] [CrossRef]
- Kadu, C.A.C.; Parich, A.; Schueler, S.; Konrad, H.; Muluvi, G.F.; Eyog-Matig, O.; Muchugi, A.; Williams, V.L.; Ramamonjisoa, L.; Kapinga, C.; et al. Bioactive constituents in Prunus africana: Geographical variation throughout Africa and associations with environmental and genetic parameters. Phytochemistry 2012, 83, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Rajasree, R.S.; Sibi, P.I.; Francis, F.; William, H. Phytochemicals of Cucurbitaceae family—A review. IJPPR 2016, 8, 113–123. [Google Scholar]
- Keehn, A.; Taylor, J.; Lowe, F.C. Phytotherapy for benign prostatic hyperplasia. Curr. Urol. Rep. 2016, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.L.; Maroto, F.G. Plants as ‘chemical factories’ for the production of polyunsaturated fatty acids. Biotechnol. Adv. 2000, 18, 481–497. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, A.; Kuźma, Ł.; Wysokińska, H.; Olszewska, M.A. Application of response surface methodology for optimisation of simultaneous UHPLC-PDA determination of oleanolic and ursolic acids and standardisation of Ericaceae medicinal plants. Appl. Sci. 2016, 6, 244. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Mroczek, A. Distribution of triterpene acids and their derivatives in organs of cowberry (Vaccinium vitis-idaea L.) plant. Acta Biochim. Pol. 2007, 54, 733–740. [Google Scholar] [PubMed]
- Szakiel, A.; Pączkowski, C.; Koivuniemi, H.; Huttunen, S. Comparison of the triterpenoid content of berries and leaves of lingonberry Vaccinium vitis-idaea from Finland and Poland. J. Agric. Food Chem. 2012, 60, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Pączkowski, C.; Huttunen, S. Triterpenoid content of berries and leaves of bilberry Vaccinium myrtillus from Finland and Poland. J. Agric. Food Chem. 2012, 60, 11839–11849. [Google Scholar] [CrossRef] [PubMed]
- Wójciak-Kosior, M.; Nowak, R.; Sokołowska-Krzaczek, A.; Pietrzak, W.; Sowa, I.; Kocjan, R. Determination of oleanolic and ursolic acid in billberies (Vaccinium myrtillus L.). Curr. Issues Pharm. Med. Sci. 2012, 25, 146–148. [Google Scholar] [CrossRef]
- Rogachev, A.D.; Komarova, N.I.; Morozov, S.V.; Fomenko, V.V.; Salakhutdinov, N.F. Phytochemical studies of Rhododendron adamsii Rehder. Quantitative determination of ursolic and oleanolic acids in some representatives of Ericaceae family. Chem. Suistain. Dev. 2007, 15, 561–564. [Google Scholar]
- Piwowarski, J.; Kiss, A.K.; Kozłowska-Wojciechowska, M. Anti-hyaluronidase and anti-elastase activity screening of tannin-rich plant materials used in traditional Polish medicine for external treatment of diseases with inflammatory background. J. Ethnopharmacol. 2011, 137, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Granica, S.; Czerwińska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical composition, antioxidant and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera paradoxa Hudziok obtained after seed cultivation. J. Agric. Food Chem. 2016, 61, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the dry leaf extracts are available from the authors.



| No. | Compound | Rta | Molecular | Q b | Relative Content (%) | ||
|---|---|---|---|---|---|---|---|
| Weight | Formula | PE c | CHE d | ||||
| 1 | methyl salicylate (SM) e | 7.84 | 152 | C8H8O3 | 100 | 2.31 | 6.88 |
| 2 | trimethylsilanol g | 9.01 | 90 | C3H10OSi | 99 | 0.86 | |
| 3 | methyl benzoate (BM) g | 10.53 | 136 | C8H8O2 | 91 | 21.59 | 10.03 |
| 4 | 4-hydroxyphenylethanol g | 12.07 | 456 | C8H10O2 | 99 | 2.78 | |
| 5 | n-dodecanoic acid (lauric acid) g | 12.61 | 200 | C12H24O2 | 99 | 0.32 | |
| 6 | m-methoxybenzoic acid (m-anisic acid) g | 13.39 | 152 | C8H8O3 | 98 | 3.60 | |
| 7 | (6S,9R)-vomifoliol (VO) e | 13.72 | 220 | C13H20O3 | 100 | 4.35 | |
| 8 | neophytadiene g | 13.79 | 278 | C20H38 | 99 | 3.44 | 5.36 |
| 9 | n-hexadecanoic acid (palmitic acid) g | 14.86 | 256 | C18H36O2 | 99 | 1.42 | 2.19 |
| 10 | phytol g | 15.49 | 296 | C20H40O | 98 | 0.69 | |
| 11 | n-octadecanoic acid (stearinic acid) g | 15.79 | 284 | C18H36O2 | 96 | 0.21 | |
| 12 | pentacosane g | 16.86 | 352 | C25H52 | 99 | 0.48 | |
| 13 | heneicosane g | 17.25 | 296 | C21H44 | 97 | 0.34 | |
| 14 | n-docosanoic acid (behenic acid) g | 17.42 | 340 | C22H44O2 | 94 | 0.19 | |
| 15 | heptacosane g | 17.69 | 380 | C27H56 | 99 | 2.40 | |
| 16 | tetracosan-1-ol g | 17.89 | 354 | C24H40O | 98 | 0.06 | |
| 17 | hexacos-1-ene g | 18.14 | 364 | C26H52 | 93 | 2.14 | |
| 18 | 13-docosenamide (erucamide) g | 18.15 | 337 | C22H43NO | 97 | 3.43 | |
| 19 | n-tetracosanoic acid (lignoceric acid) g | 18.35 | 368 | C24H48O2 | 99 | 0.02 | |
| 20 | squalene g | 18.38 | 410 | C30H50 | 99 | 0.53 | |
| 21 | tetracosane g | 18.69 | 278 | C24H50 | 94 | 0.38 | |
| 22 | octacosane g | 18.73 | 394 | C28H58 | 99 | 11.72 | |
| 23 | hexacosan-1-ol g | 18.96 | 382 | C26H54O | 90 | 0.31 | |
| 24 | hexadecane g | 19.31 | 226 | C16H34 | 95 | 1.62 | |
| 25 | nonacosane g | 20.03 | 278 | C29H60 | 96 | 0.66 | |
| 26 | 8-demethyllatifolin (DL) e | 20.12 | 328 | C18H16O6 | 100 | 1.13 | |
| 27 | docosane (DOC) g | 20.14 | 310 | C22H46 | 100 | 18.86 | |
| 28 | heptacosan-1-ol g | 20.24 | 396 | C27H56O | 94 | 0.05 | |
| 29 | octacosan-1-ol g | 20.39 | 410 | C28H58O | 91 | 0.28 | |
| 30 | stigmasta-3,5-diene g | 20.51 | 396 | C29H48 | 90 | 0.11 | |
| 31 | α-tocopherol g | 20.60 | 430 | C29H50O2 | 99 | 0.27 | |
| 32 | 8-demethylsideroxylin (DS) e | 20.89 | 298 | C17H14O5 | 100 | 2.25 | |
| 33 | n-hexacosanoic acid (cerotic acid) g | 21.27 | 396 | C26H52O2 | 90 | 0.18 | |
| 34 | tritriacontane g | 21.96 | 464 | C33H68 | 98 | 2.46 | |
| 35 | campesterol g | 22.01 | 400 | C28H48O | 97 | 0.01 | |
| 36 | β-sitosterol (β-SIT) g | 23.08 | 414 | C29H50O | 100 | 2.68 | |
| 37 | β-amyrin f | 23.35 | 426 | C30H50O | 100 | 0.98 | |
| 38 | α-amyrin f | 23.89 | 426 | C30H50O | 100 | 3.86 | |
| 39 | oleanolic acid (OA) e | 26.72 | 456 | C30H48O3 | 100 | 1.70 | 10.11 |
| 40 | ursolic acid (UA) e | 27.67 | 456 | C30H48O3 | 100 | 4.27 | 28.82 |
| Total: | 86.36 | 81.97 | |||||
| Carbon | Compound DL (CDCl3) | Compound DS (CDCl3) | ||
|---|---|---|---|---|
| δH (ppm) a | δC (ppm) a | δH (ppm) a | δC (ppm) a | |
| 2 | 155.5 | 155.7 | ||
| 3 | 139.1 | 6.58 (1H, s) | 101.1 | |
| 4 | 178.7 | 177.9 | ||
| 5 | 158.3 | 157.3 | ||
| 6 | 108.7 | 108.3 | ||
| 7 | 163.5 | 163.1 | ||
| 8 | 6.38 (1H, s) | 89.1 | 6.42 (1H, s) | 90.2 |
| 9 | 155.0 | 154.6 | ||
| 10 | 105.9 | 104.8 | ||
| 1′ | 123.4 | 122.6 | ||
| 2′, 6′ | 7.97 (2H, d, J = 8.7 Hz) | 130.4 | 7.74 (2H, d, J = 8.7 Hz) | 130.2 |
| 3′, 5′ | 6.89 (2H, d, J = 8.7 Hz) | 115.6 | 6.91 (2H, d, J = 8.7 Hz) | 115.6 |
| 4′ | 157.8 | 159.3 | ||
| 11 | 2.05 (3H, s) | 7.2 | 2.05 (3H, s) | 7.2 |
| 12 | 3.79 (3H, s) | 60.1 | 3.86 (3H, s) | 59.7 |
| 13 | 3.84 (3H, s) | 55.9 | – | |
| Carbon | Compound VO (CDCl3) | |
|---|---|---|
| δH (ppm) a | δC (ppm) a | |
| 1 | 41.2 | |
| 2a | 2.24 (1H, d, J = 16.9 Hz) | 49.7 |
| 2b | 2.44 (1H, d, J = 16.9 Hz) | |
| 3 | 197.9 | |
| 4 | 5.90 (1H, br s) | 126.9 |
| 5 | 162.6 | |
| 6 | 79.1 | |
| 7 | 5.79 (1H, dd, J1 = 0.8 Hz, J2 = 15.8 Hz) | 129.0 |
| 8 | 5.85 (1H, dd, J1 = 5.3 Hz, J2 = 15.8 Hz) | 135.8 |
| 9 | 4.40 (1H, dq, J1 = 5.3 Hz, J2 = 6.4 Hz) | 68.0 |
| 10 | 1.30 (3H, d, J = 6.4 Hz) | 23.8 |
| 11 | 1.89 (3H, d, J = 1.1 Hz) | 18.9 |
| 12 | 1.01 (3H, s) | 22.9 |
| 13 | 1.08 (3H, s) | 24.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, P.; Owczarek, A.; Matczak, M.; Kosno, M.; Szymański, P.; Mikiciuk-Olasik, E.; Kilanowicz, A.; Wesołowski, W.; Olszewska, M.A. Metabolite Profiling of Eastern Teaberry (Gaultheria procumbens L.) Lipophilic Leaf Extracts with Hyaluronidase and Lipoxygenase Inhibitory Activity. Molecules 2017, 22, 412. https://doi.org/10.3390/molecules22030412
Michel P, Owczarek A, Matczak M, Kosno M, Szymański P, Mikiciuk-Olasik E, Kilanowicz A, Wesołowski W, Olszewska MA. Metabolite Profiling of Eastern Teaberry (Gaultheria procumbens L.) Lipophilic Leaf Extracts with Hyaluronidase and Lipoxygenase Inhibitory Activity. Molecules. 2017; 22(3):412. https://doi.org/10.3390/molecules22030412
Chicago/Turabian StyleMichel, Piotr, Aleksandra Owczarek, Magdalena Matczak, Martyna Kosno, Paweł Szymański, Elżbieta Mikiciuk-Olasik, Anna Kilanowicz, Wiktor Wesołowski, and Monika A. Olszewska. 2017. "Metabolite Profiling of Eastern Teaberry (Gaultheria procumbens L.) Lipophilic Leaf Extracts with Hyaluronidase and Lipoxygenase Inhibitory Activity" Molecules 22, no. 3: 412. https://doi.org/10.3390/molecules22030412
APA StyleMichel, P., Owczarek, A., Matczak, M., Kosno, M., Szymański, P., Mikiciuk-Olasik, E., Kilanowicz, A., Wesołowski, W., & Olszewska, M. A. (2017). Metabolite Profiling of Eastern Teaberry (Gaultheria procumbens L.) Lipophilic Leaf Extracts with Hyaluronidase and Lipoxygenase Inhibitory Activity. Molecules, 22(3), 412. https://doi.org/10.3390/molecules22030412