Influence of Se/N Codoping on the Structural, Optical, Electronic and Photocatalytic Properties of TiO2
Abstract
:1. Introduction
2. Experimental and Computational Details
2.1. Materials
2.2. Preparation of Se/N-Codoped TiO2
2.3. Characterization Techniques
2.4. Photocatalytic Experiments
2.5. Computational Models and Methodology
3. Results and Discussion
3.1. Crystal Structure
3.2. Morphological Structure
3.3. Optical Absorption and Band Gap Energies
3.4. XPS Analyses
3.5. Photocatalytic Activity
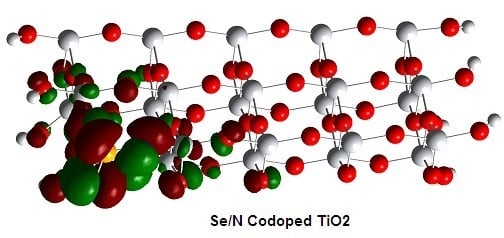
3.6. Electronic Structures
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bahnemann, D.; Cunningham, J.; Fox, M.A.; Pelizzetti, E.; Pichat, P.; Serpone, N. Aquatic and Surface Photochemistry; Lewis Publishers: Baca Raton, FL, USA, 1994; p. 261. [Google Scholar]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Pichat, P. (Ed.) Photocatalysis and Water Purification; Wiley-VCH: Weinheim, Germany, 2013.
- Schneider, J.; Bahnemann, D.; Ye, J.; Puma, L.G.; Dionysios, D.D. (Eds.) Photocatalysis: Fundamentals and Perspectives; Royal Society of Chemistry: London, UK, 2016.
- Ollis, D.F.; Pelizzetti, E.; Serpone, N. Photocatalyzed Destruction of Water Contaminants. Environ. Sci. Technol. 1991, 25, 1522–1529. [Google Scholar] [CrossRef]
- Bahnemann, D.; Bockelmann, D.; Goslich, R. Mechanistic Studies of Water Detoxification in Illuminated TiO2 Suspensions. Sol. Energy Mater. 1991, 24, 564–583. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Introduction to Photocatalysis: From Basic Science to Applications; Royal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Suib, S.L. (Ed.) New and Future Developments in Catalysis: Solar Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2013; Volume 7.
- Kilic, M.; Cinar, Z. A Quantum Mechanical Approach to TiO2 Photocatalysis. J. Adv. Oxid. Technol. 2009, 12, 37–46. [Google Scholar]
- Zhu, J.; Chen, F.; Zhang, J.; Chen, H.; Anpo, M. Fe3+-TiO2 Photocatalysts prepared by Combining Sol–Gel Method with Hydrothermal Treatment and their Characterization. J. Photochem. Photobiol. A 2006, 180, 196–204. [Google Scholar] [CrossRef]
- Yalcin, Y.; Kilic, M.; Cinar, Z. Fe+3-doped TiO2: A Combined Experimental and Computational Approach to the Evaluation of Visible Light Activity. Appl. Catal. B 2010, 99, 469–477. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Nagaveni, K.; Hegde, M.S.; Madras, G. Structure and Photocatalytic Activity of Ti1-xMxO2±δ (M = W, V, Ce, Zr, Fe, and Cu) Synthesized by Solution Combustion Method. Phys. Chem. B 2004, 108, 20204–20212. [Google Scholar] [CrossRef]
- Di Paola, A.; Marcì, G.; Palmisano, L.; Schiavello, M.; Uosaki, K.; Ikeda, S.; Ohtani, B. Preparation of Polycrystalline TiO2 Photocatalysts Impregnated with Various Transition Metal Ions: Characterization and Photocatalytic Activity for the Degradation of 4-nitrophenol. Phys. Chem. B 2002, 106, 637–645. [Google Scholar] [CrossRef]
- Mu, W.; Herrmann, J.-M.; Pichat, P. Room Temperature Photocatalytic Oxidation of Liquid Cyclohexane into Cyclohexanone over Neat and Modified TiO2. Catal. Lett. 1989, 3, 73–84. [Google Scholar] [CrossRef]
- Karakitsou, K.E.; Verykios, X.E. Effects of Altervalent Cation Doping of Titania on its Performance as a Photocatalyst for Water Cleavage. J. Phys. Chem. 1993, 97, 1184–1189. [Google Scholar] [CrossRef]
- Jagadale, T.C.; Takale, S.P.; Sonawane, R.S.; Joshi, H.M.; Patil, S.I.; Kale, B.B.; Ogale, S.B. N-doped TiO2 Nanoparticle Based Visible Light Photocatalyst by Modified Peroxide Sol−Gel Method. J. Phys. Chem. C 2008, 112, 14595–14602. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight Photocatalysis by Carbon-Modified Titanium Dioxide. Angew. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Akiyoshi, M.; Umebayashi, T.; Asai, K.; Mitsui, T.; Matsumura, M. Preparation of S-Doped TiO2 Photocatalysts and their Photocatalytic Activities under Visible Light. Appl. Catal. A 2004, 265, 115–121. [Google Scholar] [CrossRef]
- Zheng, R.; Lin, L.; Xie, J.; Zhu, Y.; Xie, Y. State of Doped Phosphorus and its Influence on the Physicochemical and Photocatalytic Properties of P-Doped Titania. J. Phys. Chem. C 2008, 112, 15502–15509. [Google Scholar] [CrossRef]
- Lu, N.; Zhao, H.; Li, J.; Quan, X.; Chen, S. Characterization of Boron-Doped TiO2 Nanotube Arrays Prepared by Electrochemical Method and its Visible Light Activity. Sep. Purif. Technol. 2008, 62, 668–673. [Google Scholar] [CrossRef]
- Yalcin, Y.; Kilic, M.; Cinar, Z. The Role of Non-Metal Doping in TiO2 Photocatalysis. J. Adv. Oxid. Technol. 2010, 13, 281–296. [Google Scholar]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of Paramagnetic Species in N-doped TiO2 Powders by EPR Spectroscopy and DFT Calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.; Janczarek, M.; Kisch, H. Visible Light Activity and Photoelectrochemical Properties of Nitrogen-Doped TiO2. J. Phys. Chem. B 2004, 108, 19384–19387. [Google Scholar] [CrossRef]
- Sato, S.; Nakamura, R.; Abe, S. Visible-Light Sensitization of TiO2 Photocatalysts by Wet-Method N Doping. Appl. Catal. A 2005, 284, 131–137. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P.; Gopinath, C.S. Synthesis, Characterization, Electronic Structure, and Photocatalytic Activity of Nitrogen-Doped TiO2 Nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Emeline, A.V.; Kuzmin, G.N.; Serpone, N. Wavelength-Dependent Photostimulated Adsorption of Molecular O2 and H2 on Second Generation Titania Photocatalysts: The Case of the Visible-Light-Active N-doped TiO2 System. Chem. Phys. Lett. 2008, 454, 279–283. [Google Scholar] [CrossRef]
- Choi, H.; Antoniou, M.G.; Pelaez, M.; de la Cruz, A.A.; Shoemaker, J.A.; Dionysiou, D.D. Mesoporous Nitrogen-Doped TiO2 for the Photocatalytic Destruction of the Cyanobacterial Toxin Microcystin-lr under Visible Light Irradiation. Environ. Sci. Technol. 2007, 41, 7530–7535. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Bai, Y.; Cheng, Y.; Jin, W.; Xu, N. Preparation and Characterization of Visible-Light-Driven Carbon-Sulfur-Codoped TiO2 Photocatalysts. Ind. Eng. Chem. Res. 2006, 45, 4971–4976. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, M.; Cheng, B.; Zhao, X. Preparation, Characterization and Photocatalytic Activity of in situ N,S-Codoped TiO2 Powders. J. Mol. Catal. A Chem. 2006, 246, 176–184. [Google Scholar] [CrossRef]
- Wang, X.; Lim, T.-T. Solvothermal Synthesis of C–N Codoped TiO2 and Photocatalytic Evaluation for Bisphenol a Degradation using a Visible-Light Irradiated LED Photoreactor. Appl. Catal. B 2010, 100, 355–364. [Google Scholar] [CrossRef]
- Li, D.; Ohashi, N.; Hishita, S.; Kolodiazhnyi, T.; Haneda, H. Origin of Visible-Light-Driven Photocatalysis: A Comparative Study on N/F-Doped and N–F-Codoped TiO2 Powders by means of Experimental Characterizations and Theoretical Calculations. J. Solid State Chem. 2005, 178, 3293–3302. [Google Scholar] [CrossRef]
- Katsanaki, A.V.; Kontos, A.G.; Maggos, T.; Pelaez, M.; Likodimos, V.; Pavlatou, E.A.; Dionysiou, D.D.; Falaras, P. Photocatalytic Oxidation of Nitrogen Oxides on N-F-Doped Titania Thin Films. Appl. Catal. B 2013, 140–141, 619–625. [Google Scholar] [CrossRef]
- Ling, Q.; Sun, J.; Zhou, Q. Preparation and Characterization of Visible-Light-Driven Titania Photocatalyst Co-Doped with Boron and Nitrogen. Appl. Surf. Sci. 2008, 254, 3236–3241. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, G.; Liu, S.; Ang, H.M.; Tadé, M.O.; Wang, S. Visible Light Responsive Titania Photocatalysts Codoped by Nitrogen and Metal (Fe, Ni, Ag, or Pt) for Remediation of Aqueous Pollutants. Chem. Eng. J. 2013, 231, 18–25. [Google Scholar] [CrossRef]
- Jaiswal, R.; Patel, N.; Kothari, D.C.; Miotello, A. Improved Visible Light Photocatalytic Activity of TiO2 Co-Doped with Vanadium and Nitrogen. Appl. Catal. B 2012, 126, 47–54. [Google Scholar] [CrossRef]
- Márquez, A.M.; Plata, J.J.; Ortega, Y.; Sanz, J.F.; Colón, G.; Kubacka, A.; Fernández-García, M. Making Photo-Selective TiO2 Materials by Cation-Anion Codoping: From Structure and Electronic Properties to Photoactivity. J. Phys. Chem. C 2012, 116, 18759–18767. [Google Scholar] [CrossRef]
- Gurkan, Y.Y.; Kasapbasi, E.; Cinar, Z. Enhanced Solar Photocatalytic Activity of TiO2 by Selenium(IV) Ion-Doping: Characterization and DFT Modeling of the Surface. Chem. Eng. J. 2013, 214, 34–44. [Google Scholar] [CrossRef]
- Gurkan, Y.Y.; Turkten, N.; Hatipoglu, A.; Cinar, Z. Photocatalytic Degradation of Cefazolin over N-doped TiO2 under UV and Sunlight Irradiation: Prediction of the Reaction Paths via Conceptual DFT. Chem. Eng. J. 2012, 184, 113–124. [Google Scholar] [CrossRef]
- Calvert, J.G.; Pitts, J.N. Photochemistry; Wiley: New York, NY, USA, 1966; pp. 783–786. [Google Scholar]
- Homann, T.; Bredow, T.; Jug, K. Adsorption of Small Molecules on the Anatase (1 0 0) Surface. Surf. Sci. 2004, 555, 135–144. [Google Scholar] [CrossRef]
- Wahab, H.S.; Bredow, T.; Aliwi, S.M. MSINDO Quantum Chemical Modeling Study of Water Molecule Adsorption at Nano-Sized Anatase TiO2 Surfaces. Chem. Phys. 2008, 354, 50–57. [Google Scholar] [CrossRef]
- Sekiya, T.; Igarashi, M.; Kurita, S.; Takekawa, S.; Fujisawa, M. Structure Dependence of Reflection Spectra of TiO2 Single Crystals. J. Electron. Spectrosc. Relat. Phenom. 1998, 92, 247–250. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Parker, J.C.; Siegel, R.W. Calibration of the Raman Spectrum to the Oxygen Stoichiometry of Nanophase TiO2. Appl. Phys. Let. 1990, 57, 943. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Krause, R.W.M.; Mamba, B.B. Nitrogen/Palladium-Codoped TiO2 for Efficient Visible Light Photocatalytic Dye Degradation. J. Phys. Chem C 2011, 115, 22110–22120. [Google Scholar] [CrossRef]
- Badrinayaran, S.; Mandale, A.B.; Gunjikar, V.G.; Sinha, A.P.B. Mechanism of High Temperature Oxidation of Tin Selenide. J. Mater. Sci. 1986, 21, 3333–3338. [Google Scholar] [CrossRef]
- Shenasa, M.; Sainkar, S.; Lichtman, D. XPS Study of Some Selected Selenium Compounds. J. Electron. Spectrosc. Relat. Phenom. 1986, 40, 329–337. [Google Scholar] [CrossRef]
- Cahen, D.; Ireland, P.J.; Kazmerski, L.L.; Thiel, F.A. X-ray Photoelectron and Auger Electron Spectroscopic Analysis of Surface Treatments and Electrochemical Decomposition of CuInSe2 Photoelectrodes. J. Appl. Phys. 1985, 57, 4761–4772. [Google Scholar] [CrossRef]
- Song, L.; Chen, C.; Zhang, S.; Wei, Q. Synthesis of Se-Doped InOOH as Efficient Visible-Light-Active Photocatalysts. Catal. Commun. 2011, 12, 1051–1054. [Google Scholar] [CrossRef]
- Emeline, A.V.; Kuznetsov, V.N.; Rybchuk, V.K.; Serpone, N. Visible-Light-Active Titania Photocatalysts: The Case of N-Doped TiO2s Properties and Some Fundamental Issues. Int. J. Photoenergy 2008, 258394. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.








| Samples | Calcination Temperature (K) 1 | Crystallite Size (nm) | λ (nm) | Eg (eV) |
|---|---|---|---|---|
| TiO2 Evonik P-25 | 623 | 22.3 | 411 | 3.01 |
| 0.25% Se–0.1% N | 623 | 19.0 | 453 | 2.73 |
| 723 | 19.3 | 442 | 2.80 | |
| 823 | 19.2 | 437 | 2.83 | |
| 0.1% Se–0.25% N | 623 | 17.9 | 460 | 2.69 |
| 723 | 18.5 | 455 | 2.72 | |
| 823 | 19.0 | 451 | 2.74 | |
| 0.5% Se–0.5% N | 623 | 16.8 | 473 | 2.62 |
| 723 | 17.4 | 467 | 2.65 | |
| 823 | 17.9 | 458 | 2.70 | |
| 0.25% Se–0.25% N | 623 | 17.3 | 482 | 2.57 |
| 723 | 17.6 | 476 | 2.60 | |
| 823 | 17.9 | 469 | 2.64 | |
| 0.1% Se–0.1% N | 623 | 19.6 | 495 | 2.50 |
| 723 | 20.1 | 488 | 2.54 | |
| 823 | 20.4 | 480 | 2.56 |
| Photocatalyst | k (10−3·min−1) | r | % Degradation |
|---|---|---|---|
| TiO2Evonik P-25 | 9.21 ± 0.009 | 0.991 | 69.83 |
| 14.15 ± 0.008 1 | 0.996 | 73.15 | |
| 0.25% Se–0.1% N | 14.85 ± 0.005 | 0.991 | 77.19 |
| 17.21 ± 0.009 | 0.998 | 79.83 | |
| 0.1% Se–0.25% N | 17.52 ± 0.008 | 0.985 | 79.58 |
| 18.89 ± 0.002 | 0.982 | 81.93 | |
| 0.5% Se–0.5% N | 20.21 ± 0.007 | 0.994 | 87.71 |
| 23.37 ± 0.006 | 0.990 | 89.25 | |
| 0.25% Se–0.25%N | 18.99 ± 0.001 | 0.987 | 82.70 |
| 20.78 ± 0.002 | 0.983 | 85.82 | |
| 0.1% Se–0.1% N | 14.97 ± 0.003 | 0.986 | 73.67 |
| 16.88 ± 0.001 | 0.995 | 75.17 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurkan, Y.Y.; Kasapbasi, E.; Turkten, N.; Cinar, Z. Influence of Se/N Codoping on the Structural, Optical, Electronic and Photocatalytic Properties of TiO2. Molecules 2017, 22, 414. https://doi.org/10.3390/molecules22030414
Gurkan YY, Kasapbasi E, Turkten N, Cinar Z. Influence of Se/N Codoping on the Structural, Optical, Electronic and Photocatalytic Properties of TiO2. Molecules. 2017; 22(3):414. https://doi.org/10.3390/molecules22030414
Chicago/Turabian StyleGurkan, Yelda Y., Esra Kasapbasi, Nazli Turkten, and Zekiye Cinar. 2017. "Influence of Se/N Codoping on the Structural, Optical, Electronic and Photocatalytic Properties of TiO2" Molecules 22, no. 3: 414. https://doi.org/10.3390/molecules22030414
APA StyleGurkan, Y. Y., Kasapbasi, E., Turkten, N., & Cinar, Z. (2017). Influence of Se/N Codoping on the Structural, Optical, Electronic and Photocatalytic Properties of TiO2. Molecules, 22(3), 414. https://doi.org/10.3390/molecules22030414