Comparison of the Sulfonamide Inhibition Profiles of the β- and γ-Carbonic Anhydrases from the Pathogenic Bacterium Burkholderia pseudomallei
Abstract
:1. Introduction
2. Results and Discussion
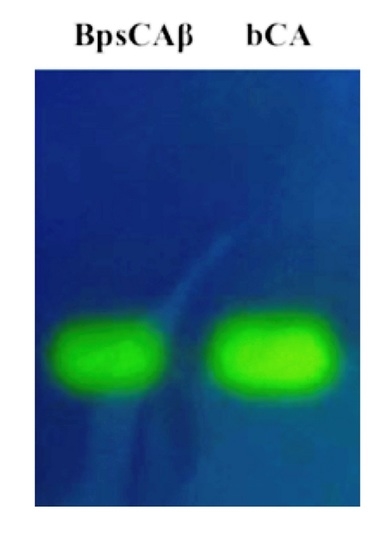
2.1. Purification and Protonographic Analysis
2.2. Kinetic Constants
2.3. Sulfonamides and Sulfamates Inhibition Profiles
3. Materials and Methods
3.1. Gene Identification and Cloning
3.2. Expression and Purification of the Recombinant BpsCAβ
3.3. Protonography
3.4. Kinetic and Inhibition Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vullo, D.; Del Prete, S.; Osman, S.M.; AlOthman, Z.; Capasso, C.; Donald, W.A.; Supuran, C.T. Burkholderia pseudomallei γ-carbonic anhydrase is strongly activated by amino acids and amines. Bioorg. Med. Chem. Lett. 2017, 27, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Del Prete, S.; De Luca, V.; Carginale, V.; Ferraroni, M.; Dedeoglu, N.; Osman, S.M.; AlOthman, Z.; Capasso, C.; Supuran, C.T. Anion inhibition studies of the β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorg. Med. Chem. Lett. 2016, 26, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Del Prete, S.; Capasso, C.; Supuran, C.T. Carbonic anhydrase activators: Activation of the β-carbonic anhydrase from Malassezia globosa with amines and amino acids. Bioorg. Med. Chem. Lett. 2016, 26, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. New light on bacterial carbonic anhydrases phylogeny based on the analysis of signal peptide sequences. J. Enzyme Inhib. Med. Chem. 2016, 31, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Ozensoy Guler, O.; Capasso, C.; Supuran, C.T. A magnificent enzyme superfamily: Carbonic anhydrases, their purification and characterization. J. Enzyme Inhib. Med. Chem. 2016, 31, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Melis, C.; Meleddu, R.; Angeli, A.; Distinto, S.; Bianco, G.; Capasso, C.; Cottiglia, F.; Angius, R.; Supuran, C.T.; Maccioni, E. Isatin: A privileged scaffold for the design of carbonic anhydrase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; Di Fonzo, P.; Osman, S.M.; AlOthman, Z.; Donald, W.A.; Supuran, C.T.; Capasso, C. Sulfonamide inhibition profile of the γ-carbonic anhydrase identified in the genome of the pathogenic bacterium Burkholderia pseudomallei the etiological agent responsible of melioidosis. Bioorg. Med. Chem. Lett. 2017, 27, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; di Fonzo, P.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Anion inhibition profiles of α-, β- and γ-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg. Med. Chem. 2016, 24, 3413–3417. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; De Luca, V.; Vullo, D.; Osman, S.M.; AlOthman, Z.; Carginale, V.; Supuran, C.T.; Capasso, C. A new procedure for the cloning, expression and purification of the β-carbonic anhydrase from the pathogenic yeast Malassezia globosa, an anti-dandruff drug target. J. Enzyme Inhib. Med. Chem. 2016, 31, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Vullo, D.; Del Prete, S.; Carginale, V.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Cloning, characterization and anion inhibition studies of a γ-carbonic anhydrase from the antarctic bacterium Colwellia psychrerythraea. Bioorg. Med. Chem. 2016, 24, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An overview of the carbonic anhydrases from two pathogens of the oral cavity: Streptococcus mutans and Porphyromonas gingivalis. Curr. Top. Med. Chem. 2016, 16, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. The η-class carbonic anhydrases as drug targets for antimalarial agents. Expert Opin. Ther. Targets 2015, 19, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; AlOthman, Z.; Osman, S.M.; Supuran, C.T.; Capasso, C. Biochemical characterization of recombinant β-carbonic anhydrase (PgiCAb) identified in the genome of the oral pathogenic bacterium Porphyromonas gingivalis. J. Enzyme Inhib. Med. Chem. 2015, 30, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Pinard, M.A.; Lotlikar, S.R.; Boone, C.D.; Vullo, D.; Supuran, C.T.; Patrauchan, M.A.; McKenna, R. Structure and inhibition studies of a type II β-carbonic anhydrase psCA3 from Pseudomonas aeruginosa. Bioorg. Med. Chem. 2015, 23, 4831–4838. [Google Scholar] [CrossRef] [PubMed]
- Ferraroni, M.; Del Prete, S.; Vullo, D.; Capasso, C.; Supuran, C.T. Crystal structure and kinetic studies of a tetrameric type II β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Monti, S.M.; Alterio, V.; Buonanno, M.; De Luca, V.; Rossi, M.; Carginale, V.; Supuran, C.T.; Capasso, C.; Di Fiore, A. Crystal structure of the most catalytically effective carbonic anhydrase enzyme known, sazca from the thermophilic bacterium Sulfurihydrogenibium azorense. Bioorg. Med. Chem. Lett. 2015, 25, 2002–2006. [Google Scholar] [CrossRef] [PubMed]
- Zolnowska, B.; Slawinski, J.; Pogorzelska, A.; Chojnacki, J.; Vullo, D.; Supuran, C.T. Carbonic anhydrase inhibitors. Synthesis, and molecular structure of novel series N-substituted N′-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl)guanidines and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Eur. J. Med. Chem. 2014, 71, 135–147. [Google Scholar] [PubMed]
- De Luca, L.; Ferro, S.; Damiano, F.M.; Supuran, C.T.; Vullo, D.; Chimirri, A.; Gitto, R. Structure-based screening for the discovery of new carbonic anhydrase VII inhibitors. Eur. J. Med. Chem. 2014, 71, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, A.; Capasso, C.; De Luca, V.; Monti, S.M.; Carginale, V.; Supuran, C.T.; Scozzafava, A.; Pedone, C.; Rossi, M.; De Simone, G. X-ray structure of the first “extremo-α-carbonic anhydrase”, a dimeric enzyme from the thermophilic bacterium Sulfurihydrogenibium yellowstonense YO3AOP1. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzyme Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases—An overview. Curr. Pharm. Des. 2008, 14, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Mahon, B.P.; Cruzeiro, V.W.; Cornelio, B.; Laronze-Cochard, M.; Ceruso, M.; Sapi, J.; Rance, G.A.; Khlobystov, A.N.; Fontana, A.; et al. Structure-activity relationships of benzenesulfonamide-based inhibitors towards carbonic anhydrase isoform specificity. Chembiochem 2017, 18, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Langella, E.; Viparelli, F.; Vullo, D.; Ascione, G.; Dathan, N.A.; Morel, F.M.; Supuran, C.T.; De Simone, G.; Monti, S.M. Structural and inhibition insights into carbonic anhydrase CDCA1 from the marine diatom Thalassiosira weissflogii. Biochimie 2012, 94, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Buzas, G.M.; Supuran, C.T. The history and rationale of using carbonic anhydrase inhibitors in the treatment of peptic ulcers. In memoriam ioan puscas (1932–2015). J. Enzyme Inhib. Med. Chem. 2016, 31, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr. Med. Chem. 2015, 22, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med. Chem. 2014, 6, 1149–1165. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An overview of the α-, β- and γ-carbonic anhydrases from bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzyme Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin. Ther. Targets 2015, 19, 1689–1704. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Sulfa and trimethoprim-like drugs—Antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J. Enzyme Inhib. Med. Chem. 2014, 29, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Anti-infective carbonic anhydrase inhibitors: A patent and literature review. Expert Opin. Ther. Pat. 2013, 23, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Ouahrani-Bettache, S.; Montero, J.L.; Nishimori, I.; Minakuchi, T.; Vullo, D.; Scozzafava, A.; Winum, J.Y.; Kohler, S.; Supuran, C.T. A new β-carbonic anhydrase from Brucella suis, its cloning, characterization, and inhibition with sulfonamides and sulfamates, leading to impaired pathogen growth. Bioorg. Med. Chem. 2011, 19, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Modak, J.K.; Liu, Y.C.; Machuca, M.A.; Supuran, C.T.; Roujeinikova, A. Structural basis for the inhibition of Helicobacter pylori α-carbonic anhydrase by sulfonamides. PLoS ONE 2015, 10, e0127149. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Onishi, S.; Takeuchi, H.; Supuran, C.T. The α and β classes carbonic anhydrases from Helicobacter pylori as novel drug targets. Curr. Pharm. Des. 2008, 14, 622–630. [Google Scholar] [PubMed]
- Nishimori, I.; Vullo, D.; Minakuchi, T.; Morimoto, K.; Onishi, S.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Cloning and sulfonamide inhibition studies of a carboxyterminal truncated α-carbonic anhydrase from Helicobacter pylori. Bioorg. Med. Chem. Lett. 2006, 16, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; De Luca, V.; Del Prete, S.; Carginale, V.; Scozzafava, A.; Osman, S.M.; AlOthman, Z.; Capasso, C.; Supuran, C.T. Sulfonamide inhibition studies of the γ-carbonic anhydrase from the antarctic bacterium Colwellia psychrerythraea. Bioorg. Med. Chem. Lett. 2016, 26, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Isik, S.; Vullo, D.; De Luca, V.; Carginale, V.; Scozzafava, A.; Supuran, C.T.; Capasso, C. DNA cloning, characterization, and inhibition studies of an α-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. J. Med. Chem. 2012, 55, 10742–10748. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: From biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J. Enzyme Inhib. Med. Chem. 2013, 28, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C. Inhibition of bacterial carbonic anhydrases as a novel approach to escape drug resistance. Curr. Top. Med. Chem. 2017, 17, 1237–1248. [Google Scholar]
- Supuran, C.T. Legionella pneumophila carbonic anhydrases: Underexplored antibacterial drug targets. Pathogens 2016, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzyme Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Pantazi, E.; De Luca, V.; Panisello, A.; Folch-Puy, E.; Hotter, G.; Capasso, C.; Supuran, C.T.; Rosello-Catafau, J. Correction: Carbonic anhydrase protects fatty liver grafts against ischemic reperfusion damage. PLoS ONE 2015, 10, e0139411. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Supuran, C.T. (In)organic anions as carbonic anhydrase inhibitors. J. Inorg. Biochem. 2012, 111, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Bacterial carbonic anhydrases as drug targets: Toward novel antibiotics? Front Pharmacol 2011, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.P.; Thomas, J.H.; Ward, L.M.; Currie, B.J. Melioidosis causing critical illness: A review of 24 years of experience from the royal darwin hospital ICU. Crit. Care Med. 2016, 44, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Gilad, J.; Schwartz, D.; Amsalem, Y. Clinical features and laboratory diagnosis of infection with the potential bioterrorism agents burkholderia mallei and Burkholderia pseudomallei. Int. J. Biomed. Sci. 2007, 3, 144–152. [Google Scholar] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Comparison of the sulfonamide inhibition profiles of the α-, β- and γ-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorg. Med. Chem. Lett. 2016, 26, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; Ferraroni, M.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Sulfonamide inhibition studies of the β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorg. Med. Chem. 2016, 24, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Modak, J.K.; Liu, Y.C.; Supuran, C.T.; Roujeinikova, A. Structure-activity relationship for sulfonamide inhibition of Helicobacter pylori α-carbonic anhydrase. J. Med. Chem. 2016, 59, 11098–11109. [Google Scholar] [CrossRef] [PubMed]
- Morishita, S.; Nishimori, I.; Minakuchi, T.; Onishi, S.; Takeuchi, H.; Sugiura, T.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Cloning, polymorphism, and inhibition of β-carbonic anhydrase of Helicobacter pylori. J. Gastroenterol. 2008, 43, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Dedeoglu, N.; DeLuca, V.; Isik, S.; Yildirim, H.; Kockar, F.; Capasso, C.; Supuran, C.T. Sulfonamide inhibition study of the β-class carbonic anhydrase from the caries producing pathogen Streptococcus mutans. Bioorg. Med. Chem. Lett. 2015, 25, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Dedeoglu, N.; De Luca, V.; Isik, S.; Yildirim, H.; Kockar, F.; Capasso, C.; Supuran, C.T. Cloning, characterization and anion inhibition study of a β-class carbonic anhydrase from the caries producing pathogen Streptococcus mutans. Bioorg. Med. Chem. 2015, 23, 2995–3001. [Google Scholar] [CrossRef] [PubMed]
- Alafeefy, A.M.; Ceruso, M.; Al-Tamimi, A.M.; Del Prete, S.; Supuran, C.T.; Capasso, C. Inhibition studies of quinazoline-sulfonamide derivatives against the γ-CA (PgiCA) from the pathogenic bacterium, Porphyromonas gingivalis. J. Enzyme Inhib. Med. Chem. 2015, 30, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Del Prete, S.; Osman, S.M.; De Luca, V.; Scozzafava, A.; Alothman, Z.; Supuran, C.T.; Capasso, C. Sulfonamide inhibition studies of the γ-carbonic anhydrase from the oral pathogen Porphyromonas gingivalis. Bioorg. Med. Chem. Lett. 2014, 24, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; Scozzafava, A.; Supuran, C.T.; Capasso, C. A highly catalytically active γ-carbonic anhydrase from the pathogenic anaerobe Porphyromonas gingivalis and its inhibition profile with anions and small molecules. Bioorg. Med. Chem. Lett. 2013, 23, 4067–4071. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Sai Kumar, R.S.; Scozzafava, A.; Capasso, C.; Ferry, J.G.; Supuran, C.T. Anion inhibition studies of a β-carbonic anhydrase from Clostridium perfringens. Bioorg. Med. Chem. Lett. 2013, 23, 6706–6710. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Scozzafava, A.; Kohler, S.; Winum, J.Y.; Supuran, C.T. Inhibition of β-carbonic anhydrases from the bacterial pathogen Brucella suis with inorganic anions. J. Inorg. Biochem. 2012, 110, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Nishimori, I.; Scozzafava, A.; Kohler, S.; Winum, J.Y.; Supuran, C.T. Inhibition studies of a β-carbonic anhydrase from Brucella suis with a series of water soluble glycosyl sulfanilamides. Bioorg. Med. Chem. Lett. 2010, 20, 2178–2182. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; Di Fonzo, P.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Anion inhibition profiles of the γ-carbonic anhydrase from the pathogenic bacterium Burkholderia pseudomallei responsible of melioidosis and highly drug resistant to common antibiotics. Bioorg. Med. Chem. 2017, 25, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Nishimori, I.; Minakuchi, T.; Scozzafava, A.; Supuran, C.T. Inhibition studies with anions and small molecules of two novel β-carbonic anhydrases from the bacterial pathogen Salmonella enterica serovar typhimurium. Bioorg. Med. Chem. Lett. 2011, 21, 3591–3595. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Minakuchi, T.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Inhibition studies of the β-carbonic anhydrases from the bacterial pathogen Salmonella enterica serovar typhimurium with sulfonamides and sulfamates. Bioorg. Med. Chem. 2011, 19, 5023–5030. [Google Scholar] [CrossRef] [PubMed]
- Burghout, P.; Vullo, D.; Scozzafava, A.; Hermans, P.W.; Supuran, C.T. Inhibition of the β-carbonic anhydrase from Streptococcus pneumoniae by inorganic anions and small molecules: Toward innovative drug design of antiinfectives? Bioorg. Med. Chem. 2011, 19, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ceruso, M.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Sulfonamides incorporating fluorine and 1,3,5-triazine moieties are effective inhibitors of three β-class carbonic anhydrases from Mycobacterium tuberculosis. J. Enzyme Inhib. Med. Chem. 2014, 29, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Minakuchi, T.; Maresca, A.; Carta, F.; Scozzafava, A.; Supuran, C.T. The β-carbonic anhydrases from Mycobacterium tuberculosis as drug targets. Curr. Pharm. Des. 2010, 16, 3300–3309. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Maresca, A.; Covarrubias, A.S.; Mowbray, S.L.; Jones, T.A.; Supuran, C.T. Carbonic anhydrase inhibitors. Characterization and inhibition studies of the most active β-carbonic anhydrase from Mycobacterium tuberculosis, rv3588c. Bioorg. Med. Chem. Lett. 2009, 19, 6649–6654. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Zimmerman, S.; Ferry, J.G.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of the β-class enzyme from the methanoarchaeon Methanobacterium thermoautotrophicum (Cab) with anions. Bioorg. Med. Chem. Lett. 2004, 14, 4563–4567. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Zimmerman, S.; Ferry, J.G.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of the zinc and cobalt γ-class enzyme from the archaeon Methanosarcina thermophila with anions. Bioorg. Med. Chem. Lett. 2004, 14, 3327–3331. [Google Scholar] [PubMed]
- Vullo, D.; De Luca, V.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. The first activation study of a bacterial carbonic anhydrase (CA). The thermostable α-CA from Sulfurihydrogenibium yellowstonense YO3AOP1 is highly activated by amino acids and amines. Bioorg. Med. Chem. Lett. 2012, 22, 6324–6327. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Luca, V.D.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. The α-carbonic anhydrase from the thermophilic bacterium Sulfurihydrogenibium yellowstonense YO3AOP1 is highly susceptible to inhibition by sulfonamides. Bioorg. Med. Chem. 2013, 21, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Vullo, D.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. Anion inhibition studies of an α-carbonic anhydrase from the thermophilic bacterium Sulfurihydrogenibium yellowstonense YO3AOP1. Bioorg. Med. Chem. Lett. 2012, 22, 5630–5634. [Google Scholar] [CrossRef] [PubMed]
- Alafeefy, A.M.; Abdel-Aziz, H.A.; Vullo, D.; Al-Tamimi, A.M.; Al-Jaber, N.A.; Capasso, C.; Supuran, C.T. Inhibition of carbonic anhydrases from the extremophilic bacteria Sulfurihydrogenibium yellostonense (Sspca) and S. azorense (Sazca) with a new series of sulfonamides incorporating aroylhydrazone-, [1,2,4]triazolo[3,4-b][1,3,4]thiadiazinyl- or 2-(cyanophenylmethylene)-1,3,4-thiadiazol-3(2H)-yl moieties. Bioorg. Med. Chem. 2014, 22, 141–147. [Google Scholar] [PubMed]
- Vullo, D.; De Luca, V.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. The extremo-α-carbonic anhydrase from the thermophilic bacterium Sulfurihydrogenibium azorense is highly inhibited by sulfonamides. Bioorg. Med. Chem. 2013, 21, 4521–4525. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, A.; Vullo, D.; De Luca, V.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. The extremo-α-carbonic anhydrase (CA) from Sulfurihydrogenibium azorense, the fastest ca known, is highly activated by amino acids and amines. Bioorg. Med. Chem. Lett. 2013, 23, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; De Luca, V.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. Anion inhibition studies of the fastest carbonic anhydrase (CA) known, the extremo-ca from the bacterium Sulfurihydrogenibium azorense. Bioorg. Med. Chem. Lett. 2012, 22, 7142–7145. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; De Luca, V.; Del Prete, S.; Carginale, V.; Scozzafava, A.; Capasso, C.; Supuran, C.T. Sulfonamide inhibition studies of the γ-carbonic anhydrase from the antarctic bacterium pseudoalteromonas haloplanktis. Bioorg. Med. Chem. Lett. 2015, 25, 3550–3555. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Vullo, D.; Del Prete, S.; Carginale, V.; Scozzafava, A.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Cloning, characterization and anion inhibition studies of a new γ-carbonic anhydrase from the antarctic bacterium Pseudoalteromonas haloplanktis. Bioorg. Med. Chem. 2015, 23, 4405–4409. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; De Luca, V.; Supuran, C.T.; Capasso, C. Protonography, a technique applicable for the analysis of η-carbonic anhydrase activity. J. Enzyme Inhib. Med. Chem. 2015, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; De Luca, V.; Iandolo, E.; Supuran, C.T.; Capasso, C. Protonography, a powerful tool for analyzing the activity and the oligomeric state of the γ-carbonic anhydrase identified in the genome of Porphyromonas gingivalis. Bioorg. Med. Chem. 2015, 23, 3747–3750. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Del Prete, S.; Supuran, C.T.; Capasso, C. Protonography, a new technique for the analysis of carbonic anhydrase activity. J. Enzyme Inhib. Med. Chem. 2015, 30, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the recombinant protein (BpsCAβ) and compounds are available from the authors.



| Bacterium | CA | Characteristics | Inhibition | |||
|---|---|---|---|---|---|---|
| Class | Acronym | Pathogenicity | Disease | Sulfonamides | Anions | |
| Vibrio cholerae [8,49,50] | α | VchCAα | + | Cholera | + | + |
| β | VchCAβ | + | + | |||
| γ | VchCAγ | + | + | |||
| Helicobacter pylori [36,51,52] | α | hpαCA | + | Gastritis, gastric | + | + |
| β | hpβCA | ulcers | + | + | ||
| Streptococcus mutans [11,53,54] | β | SmuCA | + | Caries | + | + |
| Haemophilus influenzae | β | HICA | + | Influenza | − | + |
| Neisseria gonorrhoeae | α | NgoCA | + | Gonorrhea | + | + |
| Neisseria sicca | α | NsiCA | + | Septicemia | + | − |
| Porphyromonas gingivalis [11,13,55,56,57] | β | PgiCA β | + | Periodontitis, | + | + |
| γ | PgiCA γ | rheumatoid arthritis | + | + | ||
| Legionella pneumophila [42] | β | LpCA1 | + | Legionellosis | + | + |
| β | LpCA2 | + | + | |||
| Clostridium perfringens [58] | β | CpeCA | + | Food poisoning | − | + |
| Brucella suis [34,59,60] | β | bsCA 1 | + | Brucellosis | + | − |
| β | BsCA II | + | − | |||
| Burkholderia pseudomallei [1,7,61] | β | BpsCAβ | + | Melioidosis | − | + |
| γ | BpsCAγ | + | + | |||
| Salmonella enterica [62,63] | β | stCA 1 | + | Salmonellosis | + | + |
| β | stCA 2 | + | + | |||
| Streptococcus pneumoniae [64] | β | PCA | + | Pneumonia | + | + |
| Mycobacterium tuberculosis [65,66,67] | β | mtCA 1 | + | Tuberculosis | + | − |
| β | mtCA 2 | + | − | |||
| β | mtCA 3 | + | − | |||
| Methanobacterium thermoautotrophicum [68] | β | Cab | − | − | + | + |
| Methanosarcina thermophila [69] | γ | Zn-Cam | − | − | + | + |
| γ | Co-Cam | − | − | + | + | |
| Sulfurihydrogenibium yellowstonense [19,70,71,72] | α | SspCA | − | − | + | + |
| Sulfurihydrogenibium azorense [73,74,75,76] | α | SazCA | − | − | + | + |
| Colwellia psychrerythraea [10,38] | γ | CpsCA | − | − | + | + |
| Pseudoalteromonas haloplanktis [77,78] | γ | PhaCAγ | − | − | + | + |
| Enzyme | Activity Level | Class | kcat (s−1) | kcat/Km (M−1·s−1) | KI (Acetazolamide) (nM) |
|---|---|---|---|---|---|
| hCA I | moderate | α | 2.0 × 105 | 5.0 × 107 | 250 |
| hCA II | very high | α | 1.4 × 106 | 1.5 × 108 | 12 |
| BpsCAβ | moderate | β | 1.6 × 105 | 3.4 × 107 | 745 |
| BpsCAγ | moderate | γ | 5.3 × 105 | 2.5 × 107 | 149 |
| VchCAα | high | α | 8.23 × 105 | 7.0 × 107 | 6.8 |
| VchCAβ | moderate | β | 3.34 × 105 | 4.1 × 107 | 451 |
| VchCAγ | high | γ | 7.39 × 105 | 6.4 × 107 | 473 |
| hpβCA | high | β | 7.1 × 105 | 4.8 × 107 | 40 |
| Inhibitor | KI, nM * | |||
|---|---|---|---|---|
| hCA I | hCA II | BpsCAγ | BpsCAβ | |
| 1 | 45,400 | 295 | 574 | >50,000 |
| 2 | 25,000 | 240 | 1720 | 3895 |
| 3 | 28,000 | 300 | 1550 | 3170 |
| 4 | 78,500 | 320 | >50,000 | >50,000 |
| 5 | 25,000 | 170 | >50,000 | 3900 |
| 6 | 21,000 | 160 | >50,000 | >50,000 |
| 7 | 8300 | 60 | >50,000 | >50,000 |
| 8 | 9800 | 110 | 12,500 | >50,000 |
| 9 | 6500 | 40 | >50,000 | >50,000 |
| 10 | 6000 | 70 | >50,000 | 4100 |
| 11 | 5800 | 63 | 14,000 | 425 |
| 12 | 8400 | 75 | 23,500 | 307 |
| 13 | 8600 | 60 | 18,400 | 3065 |
| 14 | 9300 | 19 | 1810 | 2500 |
| 15 | 6 | 2 | 9650 | 4345 |
| 16 | 164 | 46 | 10,800 | 2215 |
| 17 | 185 | 50 | 1825 | 417 |
| 18 | 109 | 33 | 1500 | >50000 |
| 19 | 95 | 30 | 1838 | >50000 |
| 20 | 690 | 12 | 1810 | 266 |
| 21 | 55 | 80 | 1335 | 1650 |
| 22 | 21,000 | 125 | 1805 | 5200 |
| 23 | 23,000 | 133 | 1700 | 1500 |
| 24 | 24,000 | 125 | 24,500 | >50,000 |
| AAZ | 250 | 12 | 149 | 745 |
| MZA | 50 | 14 | 1595 | 186 |
| EZA | 25 | 8 | 1865 | 3850 |
| DCP | 1200 | 38 | >50,000 | 529 |
| DZA | 50,000 | 9 | 2260 | 3670 |
| BRZ | 45,000 | 3 | 1270 | 4270 |
| BZA | 15 | 9 | 653 | 185 |
| TPM | 250 | 10 | 3010 | >50,000 |
| ZNS | 56 | 35 | >50,000 | 4060 |
| SLP | 1200 | 40 | 5600 | >50,000 |
| IND | 31 | 15 | 1800 | 4375 |
| VLX | >50,000 | 43 | >50,000 | >50,000 |
| CLX | 50,000 | 21 | >50,000 | >50,000 |
| SLT | 374 | 9 | 8900 | >50,000 |
| HCT | 328 | 290 | >50,000 | 3490 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vullo, D.; Del Prete, S.; Di Fonzo, P.; Carginale, V.; Donald, W.A.; Supuran, C.T.; Capasso, C. Comparison of the Sulfonamide Inhibition Profiles of the β- and γ-Carbonic Anhydrases from the Pathogenic Bacterium Burkholderia pseudomallei. Molecules 2017, 22, 421. https://doi.org/10.3390/molecules22030421
Vullo D, Del Prete S, Di Fonzo P, Carginale V, Donald WA, Supuran CT, Capasso C. Comparison of the Sulfonamide Inhibition Profiles of the β- and γ-Carbonic Anhydrases from the Pathogenic Bacterium Burkholderia pseudomallei. Molecules. 2017; 22(3):421. https://doi.org/10.3390/molecules22030421
Chicago/Turabian StyleVullo, Daniela, Sonia Del Prete, Pietro Di Fonzo, Vincenzo Carginale, W. Alexander Donald, Claudiu T. Supuran, and Clemente Capasso. 2017. "Comparison of the Sulfonamide Inhibition Profiles of the β- and γ-Carbonic Anhydrases from the Pathogenic Bacterium Burkholderia pseudomallei" Molecules 22, no. 3: 421. https://doi.org/10.3390/molecules22030421
APA StyleVullo, D., Del Prete, S., Di Fonzo, P., Carginale, V., Donald, W. A., Supuran, C. T., & Capasso, C. (2017). Comparison of the Sulfonamide Inhibition Profiles of the β- and γ-Carbonic Anhydrases from the Pathogenic Bacterium Burkholderia pseudomallei. Molecules, 22(3), 421. https://doi.org/10.3390/molecules22030421