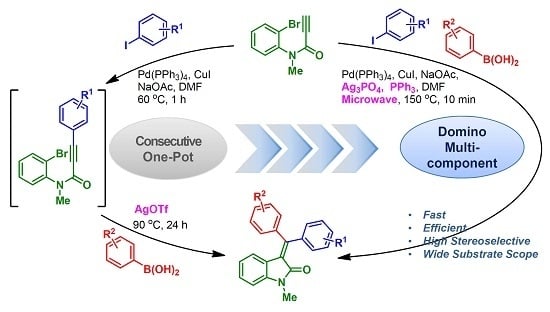
3.3. General Procedure for Microwave-Assisted Reactions
A microwave reaction vial was charged with 1 or 6 (0.15 mmol, 1.0 equiv.), aryl iodide (0.17 mmol, 1.1 equiv.), aryl boronic acid (0.18 mmol, 1.2 equiv.), CuI (0.0075 mmol, 5 mol %), NaOAc (0.45 mmol, 3.0 equiv.), Pd(PPh3)4 (0.015 mmol, 10 mol %), Ag3PO4 (0.17 mmol, 1.1 equiv.) and DMF (3 mL). The reaction vial was sealed and exposed to microwave irradiation conditions with indicated time and temperature (150 °C, 10 min; representative conditions). The mixture was cooled to 25 °C and diluted with EtOAc (100 mL). Organic layer was washed with H2O (20 mL × 3) and brine (20 mL), then dried (Na2SO4), filtered and concentrated under reduced pressure. The crude residue was purified by column chromatography (silica gel, hexane:EtOAc) to yield 3-(diarylmethylene)oxindoles 3 or 7.
3-(Diphenylmethylene)-1-methylindolin-2-one (
3a) [
22]: Yellow solid; m.p. = 165.9 °C (lit. [
22] 153.2–154.9 °C);
Rf = 0.33 (silica gel, hexanes−EtOAc 4:1); IR (film) 3054, 2923, 1700, 1606, 1469 cm
−1;
1H-NMR (CDCl
3): δ = 7.45−7.41 (m, 3H), 7.37–7.32 (m, 7H), 7.17 (td,
J = 7.5, 1.0 Hz, 1H), 6.76 (d,
J = 8.0 Hz, 1H), 6.68 (td,
J = 7.5, 1.0 Hz, 1H), 6.42 (dd,
J = 7.5, 1.0 Hz, 1H), 3.21 (s, 3H) ppm;
13C-NMR (CDCl
3): δ = 167.0, 154.7, 143.5, 141.5, 140.1, 130.1, 129.5, 129.3, 129.2, 129.1, 128.9, 128.0, 124.4, 123.4, 123.3, 121.5, 107.8, 26.0 ppm; HRMS (ESI-TOF): calcd. for C
22H
17NO [M + H
+]: 312.1388, found 312.1394.
3-(Bis(4-methoxyphenyl)methylene)-1-methylindolin-2-one (3b): Brown solid; m.p. = 192.2 °C; Rf = 0.2 (silica gel, hexanes−EtOAc 4:1); IR (film) 3017, 1691, 1603, 1251 cm−1; 1H-NMR (CDCl3): δ = 7.29–7.24 (m, 4H), 7.14 (td, J = 8.0, 1.5 Hz, 1H), 6.93 (d, J = 9.0 Hz, 2H), 6.88 (d, J = 9.0 Hz, 2H), 6.77 (d, J = 8.0 Hz, 1H), 6.70 (td, J = 8.0, 1.5 Hz, 1H), 6.57 (dd, J = 7.5, 0.5 Hz, 1H), 3.88 (s, 3H), 3.84 (s, 3H), 3.22 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 167.3, 161.0, 160.8, 155.1, 142.8, 133.9, 132.9, 132.4, 132.1, 128.0, 124.3, 122.7, 122.5, 121.3, 114.2, 113.2, 107.7, 55.5, 55.4, 26.0 ppm; HRMS (ESI-TOF): calcd. for C24H21NO3 [M + H+]: 372.1600, found 372.1611.
3-(Bis(4-chlorophenyl)methylene)-1-methylindolin-2-one (3c): Yellow solid; m.p. = 134.4 °C; Rf = 0.33 (silica gel, hexanes−EtOAc 4:1); IR (film) 3054, 2927, 1699, 1606, 1486, 1089 cm−1; 1H-NMR (CDCl3): δ = 7.41 (dd, J = 7.0, 2.0 Hz, 2H), 7.33 (dd, J = 7.0, 2.0 Hz, 2H), 7.26–7.20 (m, 5H), 6.78 (d, J = 8.0 Hz, 1H), 6.73 (td, J = 7.5, 1.0 Hz, 1H), 6.52 (d, J = 7.5 Hz, 1H), 3.20 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.7, 151.4, 143.5, 139.3, 138.0, 135.7, 135.6, 131.7, 131.1, 129.5, 129.4, 128.3, 125.1, 123.2, 122.8, 121.7, 108.1, 26.0 ppm; HRMS (ESI-TOF): calcd. for C22H15Cl2NO [M + H+]: 380.0609, found 380.0612.
3-(Bis(4-nitrophenyl)methylene)-1-methylindolin-2-one (3d): Brown solid; m.p. = 244.5 °C; Rf = 0.21 (silica gel, hexanes−EtOAc 3:1); IR (film) 3073, 1703, 1601, 1517, 1486, 1344, 1098 cm−1; 1H-NMR (CDCl3): δ = 8.34 (dd, J = 7.0, 2.0 Hz, 2H), 8.24 (dd, J = 7.0, 2.0 Hz, 2H), 7.56 (dd, J = 7.0, 2.0 Hz, 2H), 7.48 (dd, J = 7.0, 2.0 Hz, 2H), 7.29 (dd, J = 7.5, 1.0 Hz, 1H), 6.83 (d, J = 8.0 Hz, 1H), 6.76 (td, J = 8.0, 1.0 Hz, 1H), 6.45 (d, J = 7.5, 1H), 3.20 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.0, 148.4, 148.1, 147.1, 146.3, 145.6, 144.3, 130.9, 130.6, 130.4, 127.5, 124.8, 123.63, 123.61, 122.2, 121.4, 108.7, 26.1; HRMS (ESI-TOF): calcd. for C22H15 N3O5 [M + H+]: 402.1092, found 402.1097.
(E)-3-((4-Methoxyphenyl)(phenyl)methylene)-1-methylindolin-2-one (
(E)-3e) [
22]: Yellow solid; m.p. = 162.7 °C (lit. [
22] 163.8–164.3 °C);
Rf = 0.26 (silica gel, hexanes−EtOAc 4:1); IR (film) 3053, 2927, 1696, 1603, 1508, 1469, 1250 cm
−1;
1H-NMR (CDCl
3): δ = 7.39–7.25 (m, 7H), 7.17 (td,
J = 7.5, 1.5 Hz, 1H), 6.93 (dd,
J = 7.0, 2.0 Hz, 2H), 6.78–6.67 (m, 3H), 3.87 (s, 3H), 3.20 (s, 3H) ppm;
13C-NMR (CDCl
3): δ = 167.1, 160.8, 154.9, 143.3, 140.6, 133.6, 132.7, 131.7, 130.5, 129.3, 129.0, 128.6, 127.9, 122.9, 121.4, 114.3, 107.8, 55.5, 26.0 ppm; HRMS (ESI-TOF): calcd. for C
23H
19NO
2 [M + H
+]: 342.1494, found 342.1498.
(Z)-3-((4-Methoxyphenyl)(phenyl)methylene)-1-methylindolin-2-one (
(Z)-3e) [
23]: Yellow solid; m.p. = 153.0 °C (lit. [
23] 163.8–164.3 °C);
Rf = 0.24 (silica gel, hexanes−EtOAc 4:1); IR (film) 2931, 1696, 1604, 1250 cm
−1;
1H-NMR (CDCl
3): δ = 7.46–7.40 (m, 3H), 7.32–7.29 (m, 4H), 7.15 (t,
J = 13.0 Hz, 1H), 6.88 (dd,
J = 7.0, 2.0 Hz, 2H), 6.76 (d,
J = 8.0 Hz, 1H), 6.66 (t,
J = 7.5 Hz, 1H), 6.32 (d,
J = 7.5 Hz, 1H), 3.84 (s, 3H), 3.23 (s, 3H);
13C-NMR (CDCl
3): δ = 167.1, 161.0, 154.9, 143.0, 141.7, 132.7, 131.9, 129.9, 129.3, 129.0, 128.4, 123.9, 123.0, 121.4, 113.2, 107.7, 55.4, 26.0 ppm; HRMS (ESI-TOF): calcd. for C
23H
19NO
2 [M + H
+]: 342.1494, found 342.1503.
(E)-3-((4-Chlorophenyl)(phenyl)methylene)-1-methylindolin-2-one ((E)-3f): Yellow solid; m.p. = 54.8 °C; Rf = 0.35 (silica gel, hexanes−EtOAc 4:1); 1H-NMR (CDCl3): δ = 7.41–7.18 (m, 9H), 7.19 (td, J = 7.5, 1.0 Hz, 1H), 6.78 (d, J = 7.5 Hz, 1H), 6.73 (td, J = 7.5, 1.0 Hz, 1H), 6.53 (dd, J = 7.5, 0.5 Hz, 1H), 3.20 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.8, 153.0, 143.6, 139.8, 139.7, 135.5, 131.1, 130.2, 129.42, 129.37, 129.2, 128.1, 124.7, 123.2, 123.0, 121.6, 108.0, 26.0 ppm; HRMS (EI): calcd. for C22H16ClNO [M+]: 345.0920, found 345.0919.
(Z)-3-((4-Chlorophenyl)(phenyl)methylene)-1-methylindolin-2-one ((Z)-3f): Yellow solid; m.p. = 59.0 °C: Rf = 0.37 (silica gel, hexanes−EtOAc 4:1); IR (film) 3054, 1698, 1606, 1486, 1335, 1090 cm−1; 1H-NMR (CDCl3): δ = 7.48–7.43 (m, 3H), 7.35–7.27 (m, 6H), 7.17 (td, J = 7.5, 1.0 Hz, 1H), 6.77 (d, J = 8.0 Hz, 1H), 6.68 (td, J = 7.5, 1.0 Hz, 1H), 6.42 (dd, J = 7.5, 0.5 Hz, 1H), 3.20 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.9, 153.0, 143.5, 141.0, 138.4, 135.3, 131.6, 129.51, 129.50, 129.2, 129.1, 128.2, 124.7, 123.4, 123.2, 121.6, 107.9, 26.0 ppm; HRMS (EI): calcd. for C22H16ClNO [M+]: 345.0920, found 345.0916.
(E)-1-Methyl-3-((4-nitrophenyl)(phenyl)methylene)indolin-2-one (
(E)-3g) [
22]: Yellow solid; m.p. = 201.2 °C (lit. [
22] 198.8–200.8 °C);
Rf = 0.25 (silica gel, hexanes−EtOAc 4:1); IR (film) 3056, 1701, 1604, 1519, 1469, 1345 cm
−1;
1H-NMR (CDCl
3): δ = 8.30 (t,
J = 4.25 Hz, 2H), 7.54 (d,
J = 9.0 Hz, 2H), 7.41–7.21 (m, 6H), 6.80 (d,
J = 8.0 Hz, 1H), 6.71 (t,
J = 7.75 Hz, 1H), 6.36 (d,
J = 7.5 Hz, 1H), 3.21 (s, 3H) ppm;
13C-NMR (CDCl
3): δ = 166.4, 150.9, 148.2, 147.9, 143.9, 138.8, 130.6, 130.0, 129.9, 129.7, 128.3, 125.7, 124.4, 123.3, 122.4, 121.8, 108.3, 26.1 ppm; HRMS (EI): calcd. for C
22H
16N
2O
3 [M
+]: 356.1161, found 356.1161.
(Z)-1-Methyl-3-((4-nitrophenyl)(phenyl)methylene)indolin-2-one ((Z)-3g): Yellow solid; m.p. = 186.6 °C; Rf = 0.28 (silica gel, hexanes−EtOAc 4:1); IR (film) 3057, 1699, 1603, 1510, 1342 cm−1; 1H-NMR (CDCl3): δ = 8.22 (dd, J = 7.0, 1.5 Hz, 2H), 7.50–7.44 (m, 5H), 7.32 (dd, J = 8.0, 2.0 Hz, 2H), 7.22 (t, J = 7.5 Hz, 1H), 6.80 (d, J = 8.0 Hz, 1H), 6.73 (t, J = 7.75 Hz, 1H), 6.52 (d, J = 8.0 Hz, 1H), 3.20 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.6, 150.8, 147.8, 147.0, 143.9, 140.0, 130.7, 129.9, 129.8, 129.4, 129.2, 126.1, 123.7, 123.4, 122.4, 121.9, 108.2, 26.0 ppm; HRMS (ESI-TOF): calcd. for C22H16N2O3 [M + H+]: 357.1239, found 357.1249.
(E)-4-((1-Methyl-2-oxoindolin-3-ylidene)(phenyl)methyl)phenyl acetate ((E)-3h): Yellow solid; m.p. = 137.0 °C; Rf = 0.28 (silica gel, hexanes−EtOAc 4:1); IR (film) 3053, 2925, 1700, 1605, 1195 cm−1; 1H-NMR (CDCl3): δ = 7.39–7.36 (m, 3H), 7.35–7.34 (m, 2H), 7.33–7.31 (m, 2H), 7.20–7.19 (m, 1H), 7.18–7.16 (m, 2H), 6.77 (d, J = 7.8 Hz, 1H), 6.71 (td, J = 7.7, 0.8 Hz, 1H), 6.54 (d, J = 7.7 Hz, 1H), 3.20 (s, 3H), 2.34 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 169.2, 166.9, 153.6, 151.5, 143.5, 139.9, 138.7, 130.9, 130.2, 129.3, 129.0, 128.0, 124.6, 123.22, 123.20, 122.2, 121.6, 107.9, 26.0, 21.4 ppm; HRMS (EI): calcd. for C24H19NO3 [M+]: 369.1365, found 369.1366.
(Z)-4-((1-Methyl-2-oxoindolin-3-ylidene)(phenyl)methyl)phenyl acetate ((Z)-3h): Yellow solid; m.p. = 134.8 °C; Rf = 0.20 (silica gel, hexanes−EtOAc 4:1); IR (film) 3053, 2928, 1768, 1605, 1197, 1094 cm−1; 1H-NMR (CDCl3): δ = 7.46–7.41 (m, 3H), 7.37–7.35 (m, 2H), 7.32–7.30 (m, 2H), 7.16 (td, J = 7.7, 1.0 Hz, 1H), 7.11–7.08 (m, 2H), 6.77 (d, J = 7.7 Hz, 1H), 6.67 (td, J = 7.7, 0.8 Hz, 1H), 6.38 (d, J = 7.6 Hz, 1H), 3.21 (s, 3H), 2.30 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 169.3, 166.9, 153.7, 151.5, 143.4, 141.2, 137.2, 131.7, 129.6, 129.4, 129.1, 128.9, 124.5, 123.4, 123.3, 121.6, 121.0, 107.8, 26.0, 21.4 ppm; HRMS (EI): calcd. for C24H19NO3 [M+]: 369.1365, found 369.1366.
(E)-3-((3-Methoxyphenyl)(phenyl)methylene)-1-methylindolin-2-one ((E)-3i): Yellow solid; m.p. = 53.0 °C; Rf = 0.26 (silica gel, hexanes−EtOAc 4:1); IR (film) 3287, 3055, 2937, 1700, 1604, 1469, 1094, 698 cm−1; 1H-NMR (CDCl3): δ = 7.37–7.33 (m, 6H), 7.16 (td, J = 7.7, 1.1 Hz, 1H), 6.98 (ddd, J = 8.3, 2.6, 0.9 Hz, 1H), 6.77 (dt, J = 9.9, 1.9 Hz, 1H), 6.84 (dd, J = 2.5, 1.6 Hz, 1H), 6.76 (d, J = 7.8 Hz, 1H), 6.69 (td, J = 7.7, 1.0 Hz, 1H), 6.48 (d, J = 7.3 Hz, 1H), 3.77 (s, 3H), 3.20 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.9, 160.1, 154.4, 143.5, 142.7, 139.9, 130.2, 130.0, 129.2, 128.9, 128.0, 124.4, 123.5, 123.3, 121.7, 121.6, 115.0, 114.5, 107.8, 55.5, 26.0 ppm; HRMS (EI): calcd. for C23H19NO2 [M+]: 341.1416, found 341.1417.
(Z)-3-((3-Methoxyphenyl)(phenyl)methylene)-1-methylindolin-2-one ((Z)-3i): Yellow solid; m.p. = 118.2 °C; Rf = 0.21 (silica gel, hexanes−EtOAc 4:1); IR (film) 3535, 3055, 2927, 2214, 1700, 1604, 1096, 734 cm−1; 1H-NMR (CDCl3): δ = 7.43–7.41 (m, 3H), 7.33–7.29 (m, 3H), 7.15 (td, J = 7.7, 1.0 Hz, 1H), 6.95 (d, J = 7.8 Hz, 1H), 6.91 (dd, J = 8.3, 1.9 Hz, 1H), 6.83 (t, J = 1.9 Hz, 1H), 6.76 (d, J = 7.8 Hz, 1H), 6.66 (td, J = 7.7, 1.0 Hz, 1H), 6.42 (d, J = 7.6 Hz, 1H), 3.76 (s, 3H), 3.20 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.8, 159.3, 154.3, 143.5, 141.5, 141.3, 129.31, 129.27, 129.1, 128.94, 128.93, 124.5, 123.3, 122.5, 121.5, 115.7, 114.4, 107.8, 55.4, 26.0 ppm; HRMS (EI): calcd. for C23H19NO2 [M+]: 341.1416, found 341.1422.
(E)-3-((3-Chlorophenyl)(phenyl)methylene)-1-methylindolin-2-one ((E)-3j): Yellow solid; m.p. = 130.2 °C; Rf = 0.25 (silica gel, hexanes−EtOAc 4:1); IR (film) 3055, 3016, 1699, 1470, 1095 cm−1; 1H-NMR (CDCl3): δ = 7.44–7.41 (m, 1H), 7.39–7.35 (m, 4H), 7.34–7.31 (m, 3H), 7.23 (dt, J = 9.8, 1.9 Hz, 1H), 7.18 (td, J = 7.7, 1.1 Hz, 1H), 6.78 (d, J = 7.7 Hz, 1H), 6.71 (td, J = 7.7, 0.7 Hz, 1H), 6.44 (d, J = 7.7 Hz, 1H), 3.20 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.7, 152.5, 143.6, 143.1, 139.4, 135.1, 130.4, 130.0, 129.4, 129.34, 129.30, 128.1, 127.6, 125.0, 123.3, 122.9, 121.7, 108.0, 26.0 ppm; HRMS (EI): calcd. for C22H16ClNO [M+]: 345.0920, found 345.0920.
(Z)-3-((3-Chlorophenyl)(phenyl)methylene)-1-methylindolin-2-one ((Z)-3j): Yellow solid; m.p. = 143.2 °C; Rf = 0.31 (silica gel, hexanes−EtOAc 4:1); IR (film) 3055, 3016, 1699, 1607, 1470, 1098 cm−1; 1H-NMR (CDCl3): δ = 7.47–7.43 (m, 3H), 7.34–7.29 (m, 4H), 7.28–7.26 (m, 2H), 7.18 (td, J = 7.7, 1.1 Hz, 1H), 6.78 (d, J = 7.7 Hz, 1H), 6.68 (td, J = 7.7, 1.1 Hz, 1H), 6.44 (d, J = 7.7 Hz, 1H), 3.21 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.7, 152.4, 143.6, 141.9, 140.7, 134.0, 129.8, 129.5, 129.3, 129.28, 129.21, 129.1, 129.0, 128.2, 125.1, 123.4, 122.9, 121.6, 107.9, 26.0 ppm; HRMS (EI): calcd. for C22H16ClNO [M+]: 345.0920, found 345.0918.
(E)-1-Methyl-3-((3-nitrophenyl)(phenyl)methylene)indolin-2-one ((E)-3k): Yellow solid; m.p. = 186.9 °C; Rf = 0.32 (silica gel, hexanes−EtOAc 2:1); IR (film) 3055, 3017, 1699, 1528, 1350, 1096 cm−1; 1H-NMR (CDCl3): δ = 8.33–8.30 (m, 1H), 8.19 (t, J = 1.9 Hz, 1H), 7.71 (dt, J = 10.3, 1.9 Hz, 1H), 7.64 (t, J = 7.9 Hz, 1H), 7.41–7.38 (m, 3H), 7.32–7.30 (m, 2H), 7.20 (td, J = 7.7, 1.1 Hz, 1H), 6.80 (d, J = 7.8 Hz, 1H), 6.67 (td, J = 7.7, 1.0 Hz, 1H), 6.32 (d, J = 7.7 Hz, 1H), 3.21 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.4, 150.7, 148.9, 143.9, 143.0, 138.9, 135.6, 130.3, 130.0, 129.8, 129.7, 128.3, 125.8, 124.5, 124.0, 123.0, 122.4, 121.8, 108.3, 26.1 ppm; HRMS (EI): calcd. for C22H16N2O3 [M+]: 356.1161, found 356.1159.
(Z)-1-Methyl-3-((3-nitrophenyl)(phenyl)methylene)indolin-2-one ((Z)-3k): Yellow solid; m.p. = 168.1 °C; Rf = 0.28 (silica gel, hexanes−EtOAc 3:1); IR (film) 3055, 3017, 1698, 1527, 1348, 1094 cm−1; 1H-NMR (CDCl3): δ = 8.23–8.21 (m, 1H), 8.17–8.16 (m, 1H), 7.69 (d, J = 7.7 Hz, 1H), 7.53 (t, J = 8.0 Hz, 1H), 7.49–7.45 (m, 3H), 7.34–7.32 (m, 2H), 7.20 (td, J = 7.7, 0.9 Hz, 1H), 6.79 (d, J = 7.8 Hz, 1H), 6.70 (td, J = 7.7, 0.7 Hz, 1H), 6.49 (d, J = 7.7 Hz, 1H), 3.20 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.6, 150.6, 148.2, 143.8, 141.7, 140.1, 136.1, 129.84, 129.80, 129.5, 129.3, 128.9, 126.0, 125.0, 123.7, 123.6, 122.5, 121.9, 108.1, 26.1 ppm; HRMS (EI): calcd. for C22H16N2O3 [M+]: 356.1161, found 356.1159.
(E)-3-((2-Methoxyphenyl)(phenyl)methylene)-1-methylindolin-2-one ((E)-3l): Yellow solid; m.p. = 188.5 °C; Rf = 0.33 (silica gel, hexanes−EtOAc 4:1); IR (film) 2918, 2849, 1701, 1604, 1094 cm−1; 1H-NMR (CDCl3): δ = 7.44–7.40 (m, 3H), 7.36–7.32 (m, 3H), 7.21 (dd, J = 7.5, 1.5 Hz, 1H), 7.16 (t, J = 7.7 Hz, 1H), 7.05 (t, J = 7.5 Hz, 1H), 7.00 (d, J = 8.3 Hz, 1H), 6.75 (d, J = 7.8 Hz, 1H), 6.69 (t, J = 7.7 Hz, 1H), 6.23 (d, J = 7.8 Hz, 1H), 3.63 (s, 3H), 3.19 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.7, 156.4, 151.4, 143.4, 139.7, 130.5, 130.3, 129.6, 129.2, 128.7, 128.5, 127.6, 125.0, 123.5, 123.0, 121.7, 121.5, 112.2, 107.6, 55.9, 25.9 ppm; HRMS (EI): calcd. for C23H19NO2 [M+]: 341.1416, found 341.1419.
(Z)-3-((2-Methoxyphenyl)(phenyl)methylene)-1-methylindolin-2-one ((Z)-3l): Yellow solid; m.p. = 139.3 °C; Rf = 0.24 (silica gel, hexanes−EtOAc 4:1); IR (film) 3053, 2925, 2854, 1704, 1606, 1469, 1095 cm−1; 1H-NMR (CDCl3): δ = 7.40 (s, 5H), 7.34–7.31 (m, 1H), 7.19–7.14 (m, 2H), 6.97 (td, J = 7.5, 0.8 Hz, 1H), 6.93 (d, J = 8.3 Hz, 1H), 6.76 (d, J = 7.7 Hz, 1H), 6.68 (td, J = 7.7, 0.9 Hz, 1H), 6.52 (d, J = 7.7 Hz, 1H), 3.70 (s, 3H), 3.17 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.8, 156.7, 150.6, 143.6, 140.9, 130.4, 129.7, 129.6, 128.9, 128.8, 128.7, 128.6, 125.5, 123.3, 122.8, 121.4, 120.7, 111.4, 107.7, 55.8, 25.9 ppm; HRMS (EI): calcd. for C23H19NO2 [M+]: 341.1416, found 341.1419.
(E)-3-((2-Chlorophenyl)(phenyl)methylene)-1-methylindolin-2-one ((E)-3m): Yellow solid; m.p. = 164.6 °C; Rf = 0.33 (silica gel, hexanes−EtOAc 4:1); IR (film) 3053, 2918, 1703, 1605, 1095 cm−1; 1H-NMR (CDCl3): δ = 7.53–7.51 (m, 1H), 7.49–7.47 (m, 2H), 7.41–7.36 (m, 5H), 7.34–7.32 (m, 1H), 7.18 (td, J = 7.7, 1.1 Hz, 1H), 6.77 (d, J = 7.7 Hz, 1H), 6.69 (td, J = 7.7, 1.0 Hz, 1H), 6.04 (d, J = 7.7 Hz, 1H), 3.21 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.4, 150.1, 143.6, 140.0, 137.9, 132.5, 130.7, 130.0, 129.9, 129.7, 129.3, 129.1, 127.8, 125.7, 123.1, 123.0, 122.0, 107.9, 26.0 ppm; HRMS (EI): calcd. for C22H16ClNO [M+]: 345.0920, found 345.0921.
(Z)-3-((2-Chlorophenyl)(phenyl)methylene)-1-methylindolin-2-one ((Z)-3m): Yellow solid; m.p. = 199.5 °C; Rf = 0.34 (silica gel, hexanes−EtOAc 4:1); IR (film) 3054, 3017, 2931, 1706, 1606, 1099 cm−1; 1H-NMR (CDCl3): δ = 7.49–7.43 (m, 6H), 7.32–7.29 (m, 2H), 7.25–7.21 (m, 2H), 6.79 (d, J = 7.8 Hz, 1H), 6.75 (t, J = 7.6 Hz, 1H), 6.70 (d, J = 7.7 Hz, 1H), 3.19 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.6, 149.3, 144.0, 140.1, 139.4, 132.3, 129.8, 129.6, 129.4, 129.3, 129.2, 129.0, 128.9, 127.1, 126.4, 123.5, 122.1, 121.6, 108.0, 26.0 ppm; HRMS (EI): calcd. for C22H16ClNO [M+]: 345.0920, found 345.0920.
(Z)-1-Methyl-3-((2-nitrophenyl)(phenyl)methylene)indolin-2-one ((Z)-3n): Yellow solid; m.p. = 206.6 °C; Rf = 0.27 (silica gel, hexanes−EtOAc 2:1); IR (film) 3063, 3021, 2930, 1700, 1522 cm−1; 1H-NMR (CDCl3): δ = 8.15 (dd, J = 8.2, 1.0 Hz, 1H), 7.66 (td, J = 7.6, 1.1 Hz, 1H), 7.54–7.51 (m, 2H), 7.43–7.40 (m, 5H), 7.24–7.21 (m, 1H), 6.78 (d, J = 7.7 Hz, 1H), 6.75–6.74 (m, 2H), 3.15 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.8, 148.4, 147.7, 143.9, 138.1, 136.9, 133.7, 130.7, 129.7, 129.6, 129.0, 128.9, 125.2, 125.0, 123.4, 122.0, 121.8, 108.1, 26.0 ppm; HRMS (EI): calcd. for C22H16N2O3 [M+]: 356.1161, found 356.1163.
(E)-1-Methyl-3-(phenyl(pyridin-3-yl)methylene)indolin-2-one ((E)-3p): Yellow solid; m.p. = 159.0 °C; Rf = 0.24 (silica gel, hexanes−EtOAc 2:1); IR (film) 3732, 3052, 2926, 1700, 1470, 1095, 749, 698 cm−1; 1H-NMR (CDCl3): δ = 8.73 (brs, 2H), 7.62 (d, J = 7.8 Hz, 1H), 7.42–7.36 (m, 4H), 7.33–7.30 (m, 2H), 7.19 (td, J = 7.7, 1.1 Hz, 1H), 6.79 (d, J = 7.7 Hz, 1H), 6.70 (td, J = 7.7, 1.0 Hz, 1H), 6.42 (d, J = 7.5 Hz, 1H), 3.21 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.5, 150.3, 150.0, 143.7, 139.4, 137.2, 130.1, 129.5, 129.5, 128.2, 125.7, 123.0, 122.8, 121.8, 108.1, 26.0 ppm; HRMS (EI): calcd. for C21H16N2O [M+]: 312.1263, found 312.1262.
(Z)-1-Methyl-3-(phenyl(pyridin-3-yl)methylene)indolin-2-one ((Z)-3p): Yellow oil; Rf = 0.12 (silica gel, hexanes−EtOAc 2:1); 1H-NMR (CDCl3): δ = 8.58 (d, J = 17.9 Hz, 2H), 7.65 (d, J = 7.8 Hz, 1H), 7.48–7.44 (m, 3H), 7.33–7.31 (m, 3H), 7.19 (td, J = 7.7, 1.1 Hz, 1H), 6.79 (d, J = 7.7 Hz, 1H), 6.69 (td, J = 7.7, 1.0 Hz, 1H), 6.47 (d, J = 7.7 Hz, 1H), 3.21 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.9, 149.9, 143.7, 140.5, 137.4, 130.1, 129.7, 129.5, 129.4, 129.3, 125.6, 123.5, 122.8, 121.7, 108.0, 26.1 ppm: HRMS (FAB): calcd. for C21H17N2O [M + H+]: 313.1341, found 313.1342.
(E)-3-(Benzo[b]thiophen-3-yl(phenyl)methylene)-1-methylindolin-2-one ((E)-3q): Yellow solid; m.p. = 82.3 °C; Rf = 0.24 (silica gel, hexanes−EtOAc 4:1); IR (film) 3055, 3010, 2929, 1698, 1606, 1094 cm−1; 1H-NMR (CDCl3): δ = 7.93 (d, J = 8.1 Hz, 1H), 7.46–7.43 (m, 4H), 7.38–7.35 (m, 4H), 7.24 (t, J = 7.7 Hz, 1H), 7.14 (t, J = 7.7 Hz, 1H), 6.77 (d, J = 7.7 Hz, 1H), 6.60 (t, J = 7.7 Hz, 1H), 6.21 (d, J = 7.7 Hz, 1H), 3.23 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.7, 147.4, 143.4, 140.5, 139.2, 137.1, 130.0, 129.5, 129.4, 129.0, 128.0, 127.7, 125.9, 125.0, 124.9, 123.6, 123.1, 123.0, 121.8, 107.8, 26.0 ppm; HRMS (EI): calcd. for C24H17NOS [M+]: 367.1031, found 367.1033.
(Z)-3-(Benzo[b]thiophen-3-yl(phenyl)methylene)-1-methylindolin-2-one ((Z)-3q): Yellow solid; m.p. = 74.5 °C; Rf = 0.29 (silica gel, hexanes−EtOAc 4:1); IR (film) 3026, 2920, 1701, 1089, 750 cm−1; 1H-NMR (CDCl3): δ = 7.86 (d, J = 8.1 Hz, 1H), 7.48–7.41 (m, 7H), 7.33–7.30 (m, 1H), 7.25–7.20 (m, 2H), 6.80 (d, J = 7.8 Hz, 1H), 6.72 (td, J = 7.7, 1.0 Hz, 1H), 6.66 (d, J = 7.1 Hz, 1H), 3.19 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.6, 146.8, 143.6, 140.5, 140.0, 138.1, 136.4, 129.6, 129.4, 129.2, 129.1, 128.3, 126.1, 124.5, 124.4, 123.4, 123.0, 122.9, 122.8, 121.5, 107.9, 26.1 ppm; HRMS (EI): calcd. for C24H17NOS [M+]: 367.1031, found 367.1027.
(E)-3-(Benzo[b]thiophen-2-yl(phenyl)methylene)-1-methylindolin-2-one ((E)-3r): Yellow solid; m.p. = 173.7 °C; Rf = 0.27 (silica gel, hexanes:−EtOAc 4:1); IR (film) 3732, 3627, 2925, 2854, 1699, 1094, 747, 695 cm−1; 1H-NMR (CDCl3): δ = 7.84–7.79 (m, 2H), 7.50 (d, J = 0.4 Hz, 1H), 7.44–7.37 (m, 7H), 7.20 (td, J = 7.7, 1.2 Hz, 1H), 7.08 (dd, J = 7.8, 0.5 Hz, 1H), 6.79 (d, J = 7.8 Hz, 1H), 6.73 (td, J = 7.7, 1.0 Hz, 1H), 3.20 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.6, 146.3, 143.7, 143.2, 141.8, 139.7, 139.6, 130.0, 129.7, 129.6, 128.1, 126.4, 126.1, 125.5, 124.9, 124.6, 123.5, 122.9, 122.7, 121.8, 108.0, 26.1 ppm; HRMS (EI): calcd. for C24H17NOS [M+]: 367.1031, found 367.1035.
(Z)-3-(Benzo[b]thiophen-2-yl(phenyl)methylene)-1-methylindolin-2-one ((Z)-3r): Yellow solid; m.p. = 160.7 °C; Rf = 0.39 (silica gel, hexanes−EtOAc 4:1); IR (film) 3733, 3054, 2926, 1694, 1470 1419, 1373, 1092, 745, 698 cm−1; 1H-NMR (CDCl3): δ = 7.77–7.75 (m, 3H), 7.55–7.48 (m, 3H), 7.41–7.38 (m, 2H), 7.33–7.31 (m, 2H), 7.14 (td, J = 7.7, 1.1 Hz, 1H), 6.77 (d, J = 7.7 Hz, 1H), 6.62 (td, J = 7.7, 1.0 Hz, 1H), 5.97 (dd, J = 7.7, 0.5 Hz, 1H), 3.28 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.6, 146.1, 143.2, 142.5, 141.6, 141.4, 139.2, 130.4, 129.5, 129.3, 129.3, 129.0 125.7, 124.8, 124.7, 124.4, 123.9, 123.6, 122.2, 121.6, 107.8, 26.1 ppm; HRMS (EI): calcd. for C24H17NOS [M+]: 367.1031, found 367.1035.
(E)-3-(Furan-3-yl(phenyl)methylene)-1-methylindolin-2-one ((E)-3s): Yellow solid; m.p. = 122.9; Rf = 0.20 (silica gel, hexanes−EtOAc 4:1); IR (film) 3137, 3052, 2928, 1606, 1093, 740 cm−1; 1H-NMR (CDCl3): δ = 7.53 (t, J = 1.7 Hz, 1H), 7.40–7.34 (m, 7H), 7.21 (td, J = 7.7, 1.1 Hz, 1H), 6.85 (td, J = 7.7, 1.1 Hz, 1H), 6.79 (d, J = 7.7 Hz, 1H), 6.54 (dd, J = 1.8, 0.8 Hz, 1H), 3.18 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.9, 145.2, 144.7, 143.6, 143.4, 140.2, 130.2, 129.4, 128.9, 128.0, 126.8, 124.0, 123.2, 123.1, 121.5, 111.9, 107.9, 26.0 ppm; HRMS (EI): calcd. for C20H15NO2 [M+]: 301.1103, found 301.1104.
(Z)-3-(Furan-3-yl(phenyl)methylene)-1-methylindolin-2-one ((Z)-3s): Yellow solid; m.p. = 132.5; Rf = 0.37 (silica gel, hexanes−EtOAc 4:1); IR (film) 3139, 3053, 2927, 1692, 1099, 733 cm−1; 1H-NMR (CDCl3): δ = 7.73–7.72 (m, 1H), 7.50–7.47 (m, 3H), 7.40 (t, J = 1.7 Hz, 1H), 7.32–7.31 (m, 2H), 7.10 (td, J = 7.7, 1.1 Hz, 1H), 6.87 (dd, J = 1.9, 0.8 Hz, 1H), 6.75 (d, J = 7.7 Hz, 1H), 6.59 (td, J = 7.7, 1.1 Hz, 1H), 3.28 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 166.9, 148.0, 144.8, 142.8, 142.1, 141.4, 129.2, 129.1, 129.0, 128.2, 125.7, 123.9, 123.5, 122.9, 121.4, 113.1, 107.6, 26.1 ppm; HRMS (EI): calcd. for C20H15NO2 [M+]: 301.1103, found 301.1102.
4,4′-Dinitro-1,1′-biphenyl (
4d) [
24]: Yellow solid; m.p. = 235.7 °C (lit. [
24] 235–237 °C);
Rf = 0.31 (silica gel, hexanes−EtOAc 4:1);
1H-NMR (CDCl
3): δ = 8.36 (d,
J = 8.3 Hz, 4H), 7.79 (d,
J = 8.3 Hz, 4H) ppm;
13C-NMR (CDCl
3): δ = 148.2, 145.1, 128.5, 124.5 ppm.
4-Methoxy-1,1′-biphenyl (
4e) [
25]: White solid, m.p. = 86.1 °C (lit. [
25] 86–88 °C);
Rf = 0.36 (silica gel, hexanes−EtOAc 10:1);
1H-NMR (CDCl
3): δ = 7.57–7.52 (m, 4H), 7.44–7.41 (m, 2H), 7.33–7.30 (m, 1H), 7.00–6.97 (m, 2H), 3.86 (s, 3H) ppm;
13C-NMR (CDCl
3): δ = 159.2, 140.9, 133.9, 128.9, 128.3, 126.9, 126.8, 114.3, 55.5 ppm.
4-Chloro-1,1'-biphenyl (
4f) [
26]: White solid, m.p. = 69.8 °C (lit. [
26] 71–73 °C);
Rf = 0.46 (silica gel, hexane);
1H-NMR (CDCl
3): δ = 7.56–7.54 (m, 2H), 7.53–7.50 (m, 2H), 7.46–7.43 (m, 2H), 7.42–7.39 (m, 2H), 7.38–7.34 (m, 1H) ppm;
13C-NMR (CDCl
3): δ = 140.2, 139.8, 133.5, 129.1, 129.0, 128.5, 127.7, 127.1 ppm.
4-Nitro-1,1′-biphenyl (
4g) [
27]: Pale yellow solid; m.p. = 112.8 °C (lit. [
27] 112–113 °C);
Rf = 0.36 (silica gel, hexanes−EtOAc 10:1);
1H-NMR (CDCl
3): δ = 8.32–8.29 (m, 2H), 7.76–7.73 (m, 2H), 7.64–7.62 (m, 2H), 7.52–7.49 (m, 2H), 7.47–7.44 (m, 1H) ppm;
13C-NMR (CDCl
3): δ = 147.8, 147.2, 138.9, 129.3, 129.1, 127.9, 127.5, 124.3 ppm.
9-Methyl-4-phenylpyrano[2,3-b]indol-2(9H)-one (5): Yellow solid; m.p. = 146.2 °C; Rf = 0.44 (silica gel, hexanes−EtOAc 4:1); IR (film) 3055, 2924, 2853, 1732, 1527, 1470 cm−1; 1H-NMR (CDCl3): 7.65–7.64 (m, 2H), 7.57–7.54 (m, 3H), 7.35 (dd, J = 13.7, 8.1 Hz, 2H), 7.28 (td, J = 7.7, 1.1 Hz, 1H), 7.12–7.09 (m, 1H), 5.91 (s, 1H), 3.85 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 161.0, 157.2, 153.2, 136.9, 134.4, 130.1, 128.9, 128.0, 123.3, 122.0, 121.0, 120.2, 109.7, 101.4, 95.1, 28.3 ppm; HRMS (EI): calcd. for C18H13NO2 [M+]: 275.0946, found 275.0948.
(E)-3-((4-Chlorophenyl)(phenyl)methylene)indolin-2-one ((E)-7a): Dark orange solid; m.p. = 83.5 °C; Rf = 0.31 (silica gel, hexanes−EtOAc 3:2); IR (film) 32,436, 2954, 2923, 2851, 1698, 1615, 1465 cm−1; 1H-NMR (CDCl3): δ = 8.60 (brs, 1H), 7.46–7.26 (m, 9H), 7.07 (td, J = 7.7, 1.0 Hz, 1H), 6.70–6.61 (m, 2H), 6.47 (d, J = 7.8 Hz, 1H) ppm; 13C-NMR (CDCl3): 153.6, 140.6, 139.8, 139.5, 135.6, 131.2, 130.5, 129.6, 129.4, 129.2, 128.1, 124.8, 123.9, 123.4, 121.6, 109.6 ppm; HRMS (EI): calcd. for C23H18ClNO [M+]: 331.0764, found 331.0762.
(Z)-3-((4-Chlorophenyl)(phenyl)methylene)indolin-2-one one ((Z)-7a): Yellow solid; m.p. = 214.8 °C; Rf = 0.26 (silica gel, hexanes−EtOAc 2:1); IR (film) 3247, 2953, 2923, 2853, 1732, 1699, 1485 cm−1; 1H-NMR (CDCl3): δ = 8.22 (brs, 1H), 7.49–6.43 (m, 2H), 7.33–7.30 (m, 2H), 7.08 (td, J = 7.7, 1.1 Hz, 1H), 6.73 (d, J = 7.7 Hz, 1H), 6.63 (td, J = 7.7, 1.0 Hz, 1H), 6.37 (d, J = 7.8 Hz, 1H) ppm; 13C-NMR (CDCl3): δ = 168.4, 153.5, 141.0, 140.7, 138.2, 135.5, 132.0, 129.6, 129.6, 129.2, 129.2, 128.2, 124.9, 124.0, 123.6, 121.6, 109.7 ppm; HRMS (EI): calcd. for C23H18ClNO [M+]: 331.0764, found 331.0762.
(E)-1-Benzyl-3-((4-chlorophenyl)(phenyl)methylene)indolin-2-one ((E)-7b): Yellow solid; m.p. = 144.4 °C; Rf = 0.23 (silica gel, hexanes−EtOAc 10:1); IR (film) 3059, 2923, 2852, 1699, 1345 cm−1; 1H-NMR (CD2Cl2): δ = 7.47–7.44 (m, 2H), 7.42–7.31 (m, 11H), 7.30–7.26 (m, 1H), 7.08 (td, J = 7.7, 1.0 Hz, 1H), 7.47–7.43 (m, 2H), 6.53 (d, J = 7.7 Hz, 1H), 4.91 (s, 2H) ppm; 13C-NMR (CD2Cl2): δ = 166.8, 153.2, 143.0, 140.24, 140.23, 136.9, 135.6, 131.3, 130.6, 129.6, 129.5, 129.3, 129.0, 128.2, 127.8, 127.7, 124.9, 123.53, 123.46, 121.8, 109.0, 43.7 ppm; HRMS (EI): calcd. for C23H18ClNO [M+]: 421.1233, found 421.1231.
(Z)-1-Benzyl-3-((4-chlorophenyl)(phenyl)methylene)indolin-2-one ((Z)-7b): Orange solid; m.p. = 132.8 °C; Rf = 0.28 (silica gel, hexanes−EtOAc 10:1); IR (film) 3056, 2922, 2853, 1698, 1345 cm−1; 1H-NMR (CD2Cl2): δ = 7.49–7.44 (m, 3H), 7.35–7.31 (m, 10H), 7.08–7.05 (m, 1H), 7.05 (td, J = 7.7, 1.1 Hz, 1H), 6.69 (d, J = 7.8 Hz, 1H), 6.62 (td, J = 7.7, 1.0 Hz, 1H), 6.39 (dd, J = 7.7, 0.5 Hz, 1H), 4.89 (s, 2H) ppm; 13C-NMR (CD2Cl2): δ = 166.9, 153.1, 143.0, 141.4, 139.0, 137.0, 135.2, 132.1, 129.7, 129.6, 129.5, 129.2, 129.0, 128.3, 127.8, 127.7, 124.9, 123.7, 123.6, 121.8, 108.9, 43.7 ppm; HRMS (EI): calcd. for C23H18ClNO [M+]: 421.1233, found 421.1231.
(E)-4-((4-Chlorophenyl)(phenyl)methylene)-2-methyl-1,2-dihydroisoquinolin-3(4H)-one ((E)-7c): Dark orange solid; m.p. = 171.9 °C; Rf = 0.15 (silica gel, hexanes−EtOAc 3:1); IR (film) 3211, 2923, 2851, 1642, 1399 cm−1; 1H-NMR (CDCl3): δ = 7.36–7.30 (m, 4H), 7.29–7.27 (m, 1H), 7.18 (d, J = 7.0 Hz, 1H), 7.15–7.12 (m, 3H), 6.98–6.94 (m, 3H), 6.85 (d, J = 7.8 Hz, 1H), 4.49 (s, 2H), 3.03 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 165.8, 147.4, 141.7, 141.3, 134.8, 134.2, 133.8, 131.9, 130.1, 129.8, 129.6, 128.8, 127.9, 127.8, 127.27, 127.26, 125.3, 53.0, 35.3 ppm; HRMS (EI): calcd. for C23H18ClNO [M+]: 359.1077, found 359.1074.
(Z)-4-((4-Chlorophenyl)(phenyl)methylene)-2-methyl-1,2-dihydroisoquinolin-3(4H)-one ((Z)-7c): Gold solid; m.p. = 174.5 °C; Rf = 0.26 (silica gel, hexanes−EtOAc 3:1); IR (film) 3055, 2923, 2851, 1656, 1396 cm−1; 1H-NMR (CDCl3): δ = 7.33–7.35 (m, 4H), 7.18–7.15 (m, 4H), 7.11 (t, J = 7.1 Hz, 1H), 7.01–6.99 (m, 2H), 6.89 (t, J = 7.5 Hz, 1H), 6.83 (d, J = 7.8 Hz, 1H), 4.49 (s, 2H), 3.05 (s, 3H) ppm; 13C-NMR (CDCl3): δ = 165.9, 147.7, 142.4, 140.5, 131.6, 134.8, 134.0, 133.6, 131.6, 130.4, 129.9, 128.6, 128.0, 127.9, 127.2, 127.1, 125.2, 53.0, 35.3 ppm; HRMS (EI): calcd. for C23H18ClNO [M+]: 359.1077, found 359.1076.
(E)-3-((4-Chlorophenyl)(phenyl)methylene)benzofuran-2(3H)-one ((E)-7d): Yellow solid; m.p. = 145.0 °C; Rf = 0.26 (silica gel, hexanes−EtOAc 20:1); IR (film) 3359, 3188, 3058, 2922, 1783, 1460, 1074, 761 cm−1; 1H-NMR (CDCl3): δ = 7.45–7.43 (m, 3H), 7.40–7.37 (m, 2H), 7.32–7.28 (m, 4H), 7.26–7.24 (m, 1H), 7.08 (d, J = 8.0 Hz, 1H), 6.85 (td, J = 7.7, 1.0 Hz, 1H), 6.60 (dd, J = 7.8, 0.8 Hz, 1H) ppm; 13C-NMR (CDCl3): δ = 166.5, 156.8, 153.7, 138.8, 138.6, 136.3, 131.9, 131.1, 130.5, 130.4, 130.2, 129.6, 129.5, 129.3, 128.5, 128.3, 124.2, 123.4, 122.9, 119.5, 111.0 ppm; HRMS (EI): calcd. for C21H13ClO2 [M+]: 332.0604, found 332.0603.
(Z)-3-((4-Chlorophenyl)(phenyl)methylene)benzofuran-2(3H)-one ((Z)-7d): Yellow solid; m.p. = 106.4 °C; Rf = 0.31 (silica gel, hexanes−EtOAc 20:1); IR (film) 3057, 2921, 2850, 1784, 1460, 1064, 750 cm−1; 1H-NMR (CDCl3): δ = 7.51–7.49 (m, 1H), 7.48–7.45 (m, 2H), 7.36–7.34 (m, 2H), 7.32–7.30 (m, 2H), 7.29–7.27 (m, 2H), 7.22 (td, J = 7.8, 1.3 Hz, 1H), 7.07 (d, J = 7.7 Hz, 1H), 6.80 (td, J = 7.7, 1.1 Hz, 1H), 6.49 (dd, J = 7.8, 0.8 Hz, 1H) ppm; 13C-NMR (CDCl3): δ = 166.7, 156.8, 153.7, 140.1, 137.2, 136.4, 131.9, 130.3, 130.1, 129.5, 129.3, 128.5, 124.3, 123.4, 123.1, 119.6, 110.9 ppm; HRMS (EI): calcd. for C21H13ClO2 [M+]: 332.0604, found 332.0605.