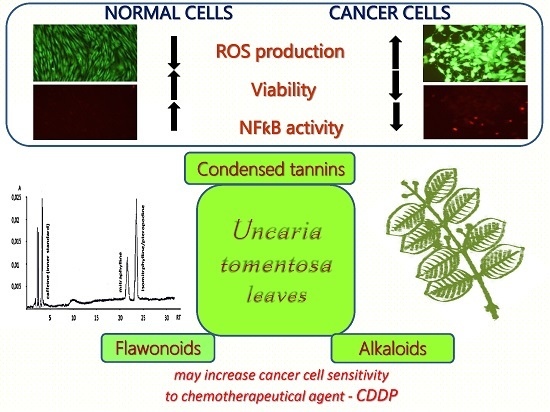
Uncaria tomentosa Leaves Decoction Modulates Differently ROS Production in Cancer and Normal Cells, and Effects Cisplatin Cytotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Composition of Studied Decoction from Uncaria tomentosa Leaves
2.2. Effect of Uncaria tomentosa Decoction on Cancer and Normal Cell Viability
2.3. Effect of Uncaria tomentosa Decoction on Cell Apoptosis
2.4. Effect of Uncaria tomentosa Decoction on ROS Production and GSH Level
2.5. Effect of Uncaria tomentosa Decoction on Caspase-3 and Caspase-7 Activity
2.6. Effect of Uncaria tomentosa Decoction on NF-κB Activity
2.7. Effect of Uncaria tomentosa on CDDP Cytotoxicity against Cancer Cells
3. Materials and Methods
3.1. Plant Material and Decoction Preparation
3.2. HPLC—Analysis of Alkaloids
3.3. Total Phenolic Content
3.4. Total Flavonoid Content
3.5. Tannin Content
3.6. Tannin Content Determined by Protein Precipitation Method
3.7. Tannin Content Determined by Vanillin/HCl Method
3.8. Cell Cultures
3.8.1. Cell Viability Assessment (MTT Assay)
3.8.2. Microscopic Examination
3.8.3. Annexin V-FITC/PI Apoptosis Assay
3.8.4. ROS Production Measurement
3.8.5. GSH Level Measurement
3.8.6. Caspase-3 and -7 Activity Detection
3.8.7. NF-κB Active form Measurement
3.8.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keplinger, K.; Laus, G.; Wurm, M.; Dierich, M.P.; Teppner, H. Uncaria tomentosa (Willd.) DC. Ethnomedicinal use and new pharmacological, toxicological and botanical results. J. Ethnopharmacol. 1999, 64, 23–34. [Google Scholar] [CrossRef]
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochemistry 2005, 66, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Shi, L.P.; Ren, Y.D.; Liu, Q.F.; Liu, H.F.; Zhang, R.J.; Li, Z.; Zhu, F.H.; He, P.L.; Tang, W.; et al. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Akesson, C.; Holmgren, K.; Bryngelsson, C.; Giamapa, V.; Pero, R.W. An active ingredient of Cat’s Claw water extracts. Identification and efficacy of quinic acid. J. Ethnopharmacol. 2005, 96, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Bors, M.; Bukowska, B.; Pilarski, R.; Gulewicz, K.; Oszmiański, J.; Michałowicz, J.; Koter-Michalak, M. Protective activity of the Uncaria tomentosa extracts on human erythrocytes in oxidative stress induced by 2,4-dichlorophenol (2,4-DCP) and catechol. Food Chem. Toxicol. 2011, 49, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Pero, R.W.; Amiri, A.; Bryngelsson, C. Induction of apoptosis and inhibition of proliferation in human tumor cells treated with extracts of Uncaria tomentosa. Anticancer Res. 1998, 18, 3363–3368. [Google Scholar] [PubMed]
- Riva, L.; Coradini, D.; Di Fronzo, G.; De Feo, V.; De Tommasi, N.; De Simone, F.; Pizza, C. The antiproliferative effects of Uncaria tomentosa extracts and fractions on the growth of breast cancer cell line. Anticancer Res. 2001, 21, 2457–2461. [Google Scholar] [PubMed]
- Cheng, A.C.; Jian, C.B.; Huang, Y.T.; Lai, C.S.; Hsu, P.C.; Pan, M.H. Induction of apoptosis by Uncaria tomentosa through reactive oxygen species production, cytochrom c release, and caspases activation in human leukemia cells. Food. Chem. Toxicol. 2007, 45, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, R.; Poczekaj-Kostrzewska, M.; Ciesiołka, D.; Szyfter, K.; Gulewicz, K. Antiproliferative activity of various Uncaria tomentosa preparations on HL-60 promyelocytic leukemia cells. Pharmacol. Rep. 2007, 59, 565–572. [Google Scholar] [PubMed]
- Pilarski, R.; Filip, B.; Wietrzyk, J.; Kuraś, M.; Gulewicz, K. Anticancer activity of the Uncaria tomentosa (Willd.) DC. Preparations with different oxindole alkaloid composition. Phytomedicine 2010, 17, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Bacher, N.; Tiefenthaler, M.; Sturm, S.; Stuppner, H.; Ausserlechner, M.J.; Kofler, R.; Konwalinka, G. Oxindole alkalois from Uncaria tomentosa induce apoptosis in proliferating, G0/G1-arrested and bcl-2-expressing acute lymphoblastic leukaemia cells. Br. J. Haematol. 2006, 132, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.G.; Gimenez, M.D.G.; Vázquez, R.P.; Sánchez, J.L.E.; Rodríguez, M.T.S. Antiproliferative effects of mitraphylline, a pentacyclic oxindole alkaloid of Uncaria tomentosa on human glioma and neuroblastoma cell lines. Phytomedicine 2007, 14, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, R.; Re, F.; Bianchi, A.; De Feo, V.; de Simone, F.; Bianchi, L.; Stivala, L.A. Mutagenic and antimutagenic activities of Uncaria tomentosa and its extracts. J. Ethnopharmacol. 1993, 38, 63–77. [Google Scholar] [CrossRef]
- Dreifuss, A.A.; Bastos-Pereira, A.L.; Fabossi, I.A.; Lívero, F.A.; Stolf, A.M.; Alves de Souza, C.E.; Gomes Lde, O.; Constantin, R.P.; Furman, A.E.; Strapasson, R.L; et al. Uncaria tomentosa exerts extensive anti-neoplastic effects against the Walker-256 tumour by modulating oxidative stress and not by alkaloid activity. PLoS ONE 2013, 8, e54618. [Google Scholar]
- Forner, A.; Hessheimer, A.J.; Real, M.I.; Bruix, J. Treatment of hepatocellular carcinoma. Crit. Rev. Oncol. Hematol. 2006, 60, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.J.; Cha, M.R.; Lee, M.R.; Yoon, M.Y.; Park, H.R. Methanolic extracts of Uncaria rhynchophylla induce cytotoxicity and apoptosis in HT-29 human colon carcinoma cells. Plant Foods Hum. Nutr. 2008, 63, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Kreutzkamp, B.; Jurcic, K. Die Alkaloide von Uncaria tomentosa und ihre Phagozytose-steigernde Wirkung. Planta Med. 1985, 51, 419–423. [Google Scholar] [CrossRef] [PubMed]
- García Giménez, D.; García Prado, E.; Sáenz Rodríguez, T.; Fernández Arche, A.; De la Puerta, R. Cytotoxic effect of the pentacyclic oxindole alkaloid mitraphylline isolated from Uncaria tomentosa bark on human Ewing’s sarcoma and breast cancer cell lines. Planta Med. 2010, 76, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Wurm, M.; Kacani, L.; Laus, G.; Keplinger, K.; Dierich, M.P. Pentacyclic oxindole alkaloids from Uncaria tomentosa induce human endothelial cells to release a lymphocyte-proliferation-regulating factor. Planta Med. 1998, 64, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Farias, I.L.; Araújo, M.C.; Farias, J.G.; Rossato, L.V.; Elsenbach, L.I.; Dalmora, S.L.; Flores, N.M.; Durigon, M.; Cruz, I.B.; Morsch, V.M.; et al. Uncaria tomentosa for Reducing Side Effects Caused by Chemotherapy in CRC Patients: Clinical Trial. Evid. Based Complement. Alternat. Med. 2012, 2012, 892182. [Google Scholar] [CrossRef] [PubMed]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [PubMed]
- Grudzinski, I.P.; Bystrzejewski, M.; Cywinska, M.A.; Kosmider, A.; Poplawska, M.; Cieszanowski, A.; Fijalek, Z.; Ostrowska, A.; Parzonko, A. Assessing carbon-encapsulated iron nanoparticles cytotoxicity in Lewis lung carcinoma cells. J. Appl. Toxicol. 2014, 34, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B. Oxidative stress, inflammation, and cancer, How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.; Charbonnet, R.M.; Okuhama, N.N.; Roberts, J.; Krenova, Z.; Trentacosti, A.M.; Miller, M.J. Cat’s claw inhibits TNFalpha production and scavenges free radicals: Role in cytoprotection. Free Radic. Biol. Med. 2000, 29, 71–78. [Google Scholar] [CrossRef]
- Akesson, C.; Lindgren, H.; Pero, R.W.; Leanderson, T.; Ivars, F. Quinic acid is a biologically active component of the Uncaria tomentosa extract C-Med 100®. Int. Immunopharmacol. 2005, 5, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Allen-Hall, L.; Arnason, J.T.; Cano, P.; Lafrenie, R.M. Uncaria tomentosa acts as a potent TNF-α inhibitor through NF-κB. J. Ethnopharmacol. 2010, 127, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Valerio, L.G.; Gonzales, G.F. Toxicological aspects of the South American herbs cat’s claw (Uncaria tomentosa) and Maca (Lepidium meyenii), a critical synopsis. Toxicol. Rev. 2005, 24, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Valls, J.; Vitrac, X.; Mérrillon, J.M.; Arola, L.; Ardèvol, A.; Bladé, C.; Fernandez-Larrea, J.; Pujadas, G.; Salvadó, J.; et al. Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting NF-κB signaling pathway. J. Agric. Food Chem. 2007, 55, 4357–4365. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Nowak, R.; Los, R.; Rzymowska, J.; Malm, A.; Chruściel, K. Biological activity and composition of teas and tinctures prepared from Rosa rugosa Thunb. Cent. Eur. J. Biol. 2012, 7, 172–182. [Google Scholar] [CrossRef]
- Lamaison, J.L.C.; Carret, A. Teneurs en principaux flavonoids des fleurs de Crataegus monogyna Jacq et de Crataegus laevigata (Piret DC) en fonction de la vegetation. Plantes Méd. Phytothér. 1990, 25, 12–16. [Google Scholar]
- Pietrzak, W.; Nowak, R.; Olech, M. Effect of extraction method on phenolic content and antioxidant activity of mistletoe extracts from Viscum album subsp. Abietis. Chem. Pap. 2014, 68, 976–982. [Google Scholar] [CrossRef]
- Polish Pharmacopoeia, 6th ed.; Polish Pharmaceutical Society: Warsaw, Poland, 2005.
- Price, N.J.; van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillic reactions in an assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Jaszewska, E.; Kośmider, A.; Kiss, A.K.; Naruszewicz, M. Pro-oxidative and pro-apoptotic action of defatted seeds of Oenothera paradoxa on human skin melanoma cells. J. Agric. Food Chem. 2009, 57, 8282–8289. [Google Scholar] [CrossRef] [PubMed]
- Grudzinski, I.P.; Bystrzejewski, M.; Cywinska, M.A.; Kosmider, A.; Poplawska, M.; Cieszanowski, A.; Fijalek, Z.; Ostrowska, A. Comparative cytotoxicity studies of carbon-encapsulated iron nanoparticles in murine glioma cells. Colloids Surf. B Biointerfaces 2014, 117, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; McCulloch, M.; Xiao, H.; Broffman, M.; Gao, J. Chinese herbal medicine and chemotherapy in the treatment of hepatocellular carcinoma: A meta-analysis of randomized controlled trials. Integr. Cancer Ther. 2005, 4, 219–229. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |











| Sample | Total Phenolic Componds TPC (mg GA/g of Dry Extract) | Total Flavonoids TFC (mg Q/g of Dry Extract) | Condensed Tannin CT | |
|---|---|---|---|---|
| Vanillin/HCl Method (mg Cat/g of Dry Extract) | Protein Precipitation Method (mg PGA/g of Dry Extract) | |||
| Dry water extract from Uncaria tomentosa leaves | 242.37 ± 0.36 | 2.26 ± 0.09 | 21.81 ± 0.41 | 110.0 ± 2.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kośmider, A.; Czepielewska, E.; Kuraś, M.; Gulewicz, K.; Pietrzak, W.; Nowak, R.; Nowicka, G. Uncaria tomentosa Leaves Decoction Modulates Differently ROS Production in Cancer and Normal Cells, and Effects Cisplatin Cytotoxicity. Molecules 2017, 22, 620. https://doi.org/10.3390/molecules22040620
Kośmider A, Czepielewska E, Kuraś M, Gulewicz K, Pietrzak W, Nowak R, Nowicka G. Uncaria tomentosa Leaves Decoction Modulates Differently ROS Production in Cancer and Normal Cells, and Effects Cisplatin Cytotoxicity. Molecules. 2017; 22(4):620. https://doi.org/10.3390/molecules22040620
Chicago/Turabian StyleKośmider, Anita, Edyta Czepielewska, Mieczysław Kuraś, Krzysztof Gulewicz, Wioleta Pietrzak, Renata Nowak, and Grażyna Nowicka. 2017. "Uncaria tomentosa Leaves Decoction Modulates Differently ROS Production in Cancer and Normal Cells, and Effects Cisplatin Cytotoxicity" Molecules 22, no. 4: 620. https://doi.org/10.3390/molecules22040620
APA StyleKośmider, A., Czepielewska, E., Kuraś, M., Gulewicz, K., Pietrzak, W., Nowak, R., & Nowicka, G. (2017). Uncaria tomentosa Leaves Decoction Modulates Differently ROS Production in Cancer and Normal Cells, and Effects Cisplatin Cytotoxicity. Molecules, 22(4), 620. https://doi.org/10.3390/molecules22040620