Investigation of the Anti-Leishmania (Leishmania) infantum Activity of Some Natural Sesquiterpene Lactones
Abstract
:1. Introduction
2. Results and Discussion
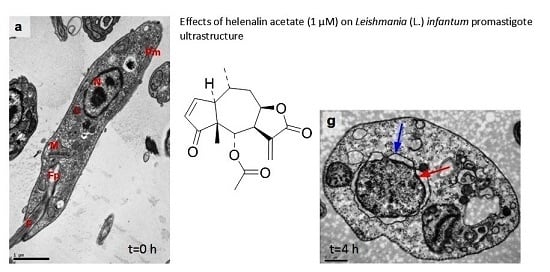
Anti-L. (L.) infantum Activity of STL
3. Materials and Methods
3.1. Compounds
3.2. Animals
3.3. Leishmania (Leishmania) infantum Promastigote, Peritoneal Macrophages, and NCTC Cells Culture
3.4. Determination of 50% Inhibitory Concentration (IC50) against Leishmania and 50% Cytotoxicity Concentration (CC50) against NCTC
3.4.1. Promastigotes
3.4.2. Intracellular Amastigotes of L. (L.) infantum
3.4.3. Cytotoxicity
3.5. Ultrastructural Analysis of Cellular Damage in Promastigotes Treated with Helenalin Acetate, Using TEM
3.6. Determination of Plasma Membrane Permeability in Helenalin Acetate-Treated Promastigotes
3.7. Determination of the Mitochondrial Membrane Potential (Δψm) and ROS by Fluorescence-Activated Cell Sorting
3.8. Production of Nitric Oxide (NO) in Macrophages Treated with Helenalin Acetate
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). The 17 Neglected Tropical Diseases. World Health Organization. Available online: http://www.who.int/neglected_diseases/diseases/en/ (accessed on 1 July 2016).
- Grimaldi, G.; Tesh, R.B. Leishmaniasis of the New World: Current concepts and implications for future research. Clin. Microbiol. Rev. 1993, 6, 230–250. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R.; Shaw, J.J. Epidemiology and ecology of leishmaniasis in Latin-America. Nature 1978, 5664, 595–600. [Google Scholar] [CrossRef]
- De Freitas, E.O.; Leoratti, F.M.; Freire-de-Lima, C.G.; Morrot, A.; Feijó, D.F. The Contribution of Immune Evasive Mechanisms to Parasite Persistence in Visceral Leishmaniasis. Front. Immunol. 2016, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Lockwood, D.N. Treatment of visceral leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 151–158. [Google Scholar] [PubMed]
- Croft, S.L.; Olliaro, P. Leishmaniasis chemotherapy-challenges and opportunities. Clin. Microbiol. Infect. 2011, 10, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Tempone, A.G.; de Oliveira, C.M.; Berlinck, R.G.S. Current approaches to discover marine antileishmanial natural products. Planta Med. 2011, 6, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Chatterjee, M. Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine 2011, 12, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.A.; Biavatti, M.W.; Brun, R.; Ferreira, V.F.; Lago, J.H.G.; Leon, L.L.; Lopes, N.P.; et al. The Potential of Secondary Metabolites from Plants as Drugs or Leads against Protozoan Neglected Diseases—Part I. Curr. Med. Chem. 2012, 14, 2128–2175. [Google Scholar]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.A.; Biavatti, M.W.; Brun, R.; Ferreira, V.F.; Lago, J.H.G.; Leon, L.L.; Lopes, N.P.; et al. The Potential of Secondary Metabolites from Plants as Drugs or Leads against Protozoan Neglected Diseases—Part II. Curr. Med. Chem. 2012, 14, 2176–2228. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 3, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Towers, G.N.H.; Mitchell, J.C. Biological activities of sesquiterpene lactones. Phytochemistry 1976, 15, 1573–1580. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Eakin, M.A.; Thomas, A.M. Tumor inhibitors. Structure-cytotoxicity relationships among the sesquiterpene lactones. J. Med. Chem. 1971, 12, 1147–1152. [Google Scholar] [CrossRef]
- Robles, M.; Aregullin, M.; West, J.; Rodriguez, E. Recent studies on the zoopharmacognosy, pharmacology and neurotoxicology of sesquiterpene lactones. Planta Med. 1995, 3, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Giordano, O.S.; Guerreiro, E.; Pestchanker, M.J.; Guzman, J.; Pastor, D.; Guardia, T. The gastric cytoprotective effect of several sesquiterpene lactones. J. Nat. Prod. 1990, 4, 803–809. [Google Scholar] [CrossRef]
- Ordóñez, P.E.; Sharma, K.K.; Bystrom, L.M.; Alas, M.A.; Enriquez, R.G.; Malagón, O.; Jones, D.E.; Guzman, M.L.; Compadre, C.M. Dehydroleucodine, a Sesquiterpene Lactone from Gynoxys verrucosa, Demonstrates Cytotoxic Activity against Human Leukemia Cells. J. Nat. Prod. 2016, 4, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Brengio, S.D.; Belmonte, S.A.; Guerreiro, E.; Giordano, O.S.; Pietrobon, E.O.; Sosa, M.A. The sesquiterpene lactone dehydroleucodine (DhL) affects the growth of cultured epimastigotes of Trypanosoma cruzi. J. Parasitol. 2000, 2, 407–412. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Brun, R.; Willuhn, G.; Khalid, S.A. Anti-trypanosomal activity of helenalin and some structurally related sesquiterpene lactones. Planta Med. 2002, 8, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Ortiz, V.; Brengio, S.D.; Giordano, O.; Tonn, C.; Sánchez, M.; Burgos, M.H.; Sosa, M.A. The trypanocidal effect of sesquiterpene lactones helenalin and mexicanin on cultured epimastigotes. J. Parasitol. 2005, 1, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Barrera, P.A.; Jimenez-Ortiz, V.; Tonn, C.; Giordano, O.; Galanti, N.; Sosa, M.A. Natural sesquiterpene lactones are active against Leishmania mexicana. J. Parasitol. 2008, 5, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Tiuman, T.S.; Ueda-Nakamura, T.; Alonso, A.; Nakamura, C.V. Cell death in amastigote forms of Leishmania amazonensis induced by parthenolide. BMC Microbiol. 2014, 10, 14–152. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R. Quantitative structure--antiprotozoal activity relationships of sesquiterpene lactones. Molecules 2009, 6, 2062–2076. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Da Costa, F.B.; Lopes, N.P.; Kaiser, M.; Brun, R. In Silico prediction and experimental evaluation of furanoheliangolide sesquiterpene lactones as potent agents against Trypanosoma brucei rhodesiense. Antimicrob. Agents Chemother. 2014, 1, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Zilberstein, D.; Dwyer, D.M. Protonmotive force-driven active transport of d-glucose and l-proline in the protozoan parasite Leishmania donovani. Proc. Natl. Acad. Sci. USA 1985, 6, 1716–1720. [Google Scholar] [CrossRef]
- Luque-Ortega, J.R.; Rivas, L. Characterization of the leishmanicidal activity of antimicrobial peptides. In Antimicrobial Peptides; Methods in Molecular Biology; Humana Press: London, UK, 2010; Volume 618, pp. 393–420. [Google Scholar]
- Carvalho, L.; Luque-Ortega, J.R.; Manzano, J.I.; Castanys, S.; Rivas, L.; Gamarro, F. Tafenoquine, an antiplasmodial 8-aminoquinoline, targets leishmania respiratory complex III and induces apoptosis. Antimicrob. Agents Chemother. 2010, 12, 5344–5351. [Google Scholar] [CrossRef] [PubMed]
- Van Hellemond, J.J.; Van Der Meer, P.; Tielens, A.G.M. Leishmania infantum promastigotes have a poor capacity for anaerobic functioning and depend mainly on respiration for their energy generation. Parasitology 1997, 114, 351–360. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 2, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; Vercesi, A.E. Mitochondrial damage induced by conditions of oxidative stress. Free Radic. Biol. Med. 1999, 3–4, 463–471. [Google Scholar] [CrossRef]
- Mehta, A.; Shaha, C. Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition: Complex II inhibition results in increased pentamidine cytotoxicity. J. Biol. Chem. 2004, 12, 11798–11813. [Google Scholar] [CrossRef] [PubMed]
- Martino, R.; Beer, M.F.; Elso, O.; Donadel, O.; Sülsen, V.; Anesini, C. Sesquiterpene lactones from Ambrosia spp. are active against a murine lymphoma cell line by inducing apoptosis and cell cycle arrest. Toxicol. In Vitro 2015, 7, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Hamzeloo-Moghadam, M.; Aghaei, M.; Fallahian, F.; Jafari, S.M.; Dolati, M.; Abdolmohammadi, M.H.; Hajiahmadi, S.; Esmaeili, S. Britannin, a sesquiterpene lactone, inhibits proliferation and induces apoptosis through the mitochondrial signaling pathway in human breast cancer cells. Tumour Biol. 2015, 2, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.Y.; Wang, S.; Li, H.Y.; Chen, W.B.; Wang, G.C.; Ma, D.L.; Wong, N.S.; Xiao, H.; Liu, Q.Y.; Zhou, G.X.; et al. EM23, a natural sesquiterpene lactone, targets thioredoxin reductase to activate JNK and cell death pathways in human cervical cancer cells. Oncotarget 2016, 6, 6790–6808. [Google Scholar]
- Barrera, P.; Sülsen, V.P.; Lozano, E.; Rivera, M.; Beer, M.F.; Tonn, C.; Martino, V.S.; Sosa, M.A. Natural Sesquiterpene Lactones Induce Oxidative Stress in Leishmania mexicana. Evid. Based Complement. Altern. Med. 2013, 2013, 163404. [Google Scholar] [CrossRef] [PubMed]
- Edinger, A.L.; Thompson, C.B. Death by design: Apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004, 6, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, V.; Carcelén, J.; Molano, I.; Peixoto, P.M.V.; Redondo, E.; Parra, P.; Mangas, M.; Monroy, I.; Campo, M.L.; Nieto, C.G.; et al. Arginase I Induction during Leishmania major Infection Mediates the Development of Disease. Infect. Immun. 2005, 9, 6085–6090. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, V.; Gómez-Nieto, L.C.; Molano, I.; Mohedano, A.; Carcelén, J.; Mirón, C.; Alonso, C.; Corraliza, I. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol. 2002, 3, 113–118. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 12, 6166–6173. [Google Scholar] [CrossRef]
- Lezama-Dávila, C.M.; Isaac-Márquez, A.P.; Barbi, J.; Cummings, H.E.; Lu, B.; Satoskar, A.R. Role of phosphatidylinositol-3-kinase-γ (PI3Kγ)-mediated pathway in 17β-estradiol-induced killing of L. mexicana in macrophages from C57BL/6 mice. Immunol. Cell Biol. 2008, 6, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Shiho, O.; Kuroshima, K.; Koyama, M.; Tsukamoto, K. An improved colorimetric assay for interleukin 2. J. Immunol. Methods 1986, 2, 157–165. [Google Scholar] [CrossRef]
- Duarte, M.I.; Mariano, O.N.; Takakura, C.F.; Everson, D.; Corbett, C.E. A fast method for processing biologic material for electron microscopic diagnosis in infectious disease. Ultrastruct. Pathol. 1992, 4, 475–482. [Google Scholar] [CrossRef]
- Pinto, E.G.; Pimenta, D.C.; Antoniazzi, M.M.; Jared, C.; Tempone, A.G. Antimicrobial peptides isolated from Phyllomedusa nordestina (Amphibia) alter the permeability of plasma membrane of Leishmania and Trypanosoma cruzi. Exp. Parasitol. 2013, 135, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, J.T.; Da Costa-Silva, T.A.; Borborema, S.E.; Tempone, A.G. Activity of imidazole compounds on Leishmania (L.) infantum chagasi: Reactive oxygen species induced by econazole. Mol. Cell. Biochem. 2014, 1–2, 293–300. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–7 are available from the authors (T.J.S.). |





| Compounds | L. (L.) infantum Promastigotes | L. (L.) infantum Amastigotes | Cytotoxicity | SI (NCTC/Amastigote) |
|---|---|---|---|---|
| IC50 (μM) (SD) | IC50 (μM) (SD) | CC50 (μM) (SD) | ||
| Helenalin acetate (1) | 3.53 (0.2) | 1.15 (0.22) | 8.12 (1.47) | 7.0 |
| Mexicanin I (2) | 4.89 (1.2) | 1.73 (0.7) | 9.20 (2.74) | 5.3 |
| Arglabin (3) | 29.98 (2.4) | 7.33 (4.0) | 39.35 (6.38) | 5.3 |
| Cynaropicrin (4) | 30.59 (0.61) | 6.88 (3.5) | 33.54 (7.39) | 4.8 |
| Alantolactone (5) | 9.94 (0.14) | n.a. | 11.04 (1.61) | - |
| Parthenolide (6) | 59.13 (4.00) | 89.20 (-) | 58.18 (9.14) | <1 |
| Budlein A (7) | 28.00 (7.87) | n.a. | 9.05 (1.86) | - |
| Miltefosine | 16.69 (3.49) | 17.80 (1.39) | 116.70 (5.30) | 6.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wulsten, I.F.; Costa-Silva, T.A.; Mesquita, J.T.; Lima, M.L.; Galuppo, M.K.; Taniwaki, N.N.; Borborema, S.E.T.; Da Costa, F.B.; Schmidt, T.J.; Tempone, A.G. Investigation of the Anti-Leishmania (Leishmania) infantum Activity of Some Natural Sesquiterpene Lactones. Molecules 2017, 22, 685. https://doi.org/10.3390/molecules22050685
Wulsten IF, Costa-Silva TA, Mesquita JT, Lima ML, Galuppo MK, Taniwaki NN, Borborema SET, Da Costa FB, Schmidt TJ, Tempone AG. Investigation of the Anti-Leishmania (Leishmania) infantum Activity of Some Natural Sesquiterpene Lactones. Molecules. 2017; 22(5):685. https://doi.org/10.3390/molecules22050685
Chicago/Turabian StyleWulsten, Imke F., Thais A. Costa-Silva, Juliana T. Mesquita, Marta L. Lima, Mariana K. Galuppo, Noemi N. Taniwaki, Samanta E. T. Borborema, Fernando B. Da Costa, Thomas J. Schmidt, and Andre G. Tempone. 2017. "Investigation of the Anti-Leishmania (Leishmania) infantum Activity of Some Natural Sesquiterpene Lactones" Molecules 22, no. 5: 685. https://doi.org/10.3390/molecules22050685
APA StyleWulsten, I. F., Costa-Silva, T. A., Mesquita, J. T., Lima, M. L., Galuppo, M. K., Taniwaki, N. N., Borborema, S. E. T., Da Costa, F. B., Schmidt, T. J., & Tempone, A. G. (2017). Investigation of the Anti-Leishmania (Leishmania) infantum Activity of Some Natural Sesquiterpene Lactones. Molecules, 22(5), 685. https://doi.org/10.3390/molecules22050685