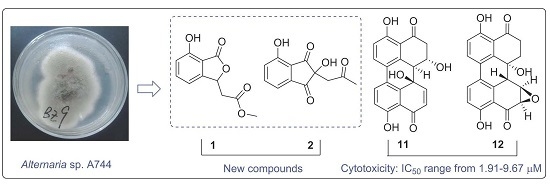
Two New Metabolites from the Endophytic Fungus Alternaria sp. A744 Derived from Morinda officinalis
Abstract
:1. Introduction
2. Results
2.1. Structural Elucidation of New Compounds
2.2. In Vitro Cytotoxicity Assay
2.3. α-Glucosidase Inhibitory Activity Assay
3. Materials and Methods
3.1. General Experimental Material
3.2. Fungal Material
3.3. Fermentation, Extraction and Compound Isolation
3.4. Spectroscopic Data
3.5. In Vitro Cytotoxicity Assay
3.6. α-Glucosidase Inhibitory Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aly, A.H.; Debbab, A.; Kjer, J.; Proksch, P. Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010, 41, 1–16. [Google Scholar] [CrossRef]
- Wang, G.W.; Huang, B.K.; Qin, L.P. The genus Broussonetia: A review of its phytochemistry and pharmacology. Phytother. Res. 2012, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Pharmacopoeia Committee of China. Pharmacopoeia of People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2015; pp. 81–82. [Google Scholar]
- Wang, M.; Sun, Z.H.; Chen, Y.C.; Liu, H.X.; Li, H.H.; Tan, G.H.; Li, S.N.; Guo, X.L.; Zhang, W.M. Cytotoxic cochlioquinone derivatives from the endophytic fungus Bipolaris sorokiniana derived from Pogostemon cablin. Fitoterapia 2016, 110, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Liang, F.L.; Wu, W.; Chen, Y.C.; Pan, Q.L.; Li, H.H.; Ye, W.; Liu, H.X.; Li, S.N.; Tan, G.H.; et al. Guignardones P-S, new meroterpenoids from the endophytic fungus Guignardia mangiferae A348 derived from the medicinal plant Smilax glabra. Molecules 2015, 20, 22900–22907. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Tan, H.B.; Liu, Y.; Chen, Y.C.; Li, S.N.; Sun, Z.H.; Li, H.H.; Qiu, S.X.; Zhang, W.M. Three new highly-oxygenated metabolites from the endophytic fungus Cytospora rhizophorae A761. Fitoterapia 2017, 117, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Li, H.H.; Liang, F.L.; Chen, Y.C.; Liu, H.X.; Li, S.N.; Tan, G.H.; Zhang, W.M. Two new secondary metabolites from the endophytic Fungus Endomelanconiopsis endophytica. Molecules 2016, 21, 943. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.A.; Upadhyay, R.; Hershenhorn, J.; Strobel, G.A.; Molina, G. The phytotoxins of Mycosphaerella fijiensis, the causative agent of Black Sigatoka disease of bananas and plantains. Experientia 1991, 47, 835–859. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Kobayash, T.; Izawa, M. Indanostatin, a new neuroprotective compound from Streptomyces sp. J. Antibiot. 2013, 66, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, T.; Veitch, N.C.; Bridge, P.D.; Simmonds, M.S.J. Dihydroisocoumarins and a tetralone from Cytospora eucalypticola. Phytochemistry 2003, 62, 779–782. [Google Scholar] [CrossRef]
- Iwasaki, S.; Muro, H.; Sasaki, K.; Nozoe, S.; Okuda, S.; Sato, Z. Isolations of phytotoxic substances produced by Pyricularia oryzae Cavara. Tetrahedron Lett. 1973, 14, 3537–3542. [Google Scholar] [CrossRef]
- Dong, J.Y.; Song, H.C.; Li, J.H.; Tang, Y.S.; Sun, R.; Wang, L.; Zhou, Y.P.; Wang, L.M.; Shen, K.Z.; Wang, C.R.; et al. Ymf 1029A-E, preussomerin analogues from the fresh-eater-derived fungus YMF 1.01029. J. Nat. Prod. 2008, 71, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.A.; Stipanovic, R.D.; Puhalla, J.E. Pentaketide metabolites of Verticillium dahlia. Identification of (+)-scytalone as anatural proecursor to melanin. Tetrahedron 1976, 32, 1353–1356. [Google Scholar] [CrossRef]
- Tan, N.; Tao, Y.; Pan, J.; Wang, S.; Xu, F. Isolation, structure elucidation, and mutagenicity of four alternariol derivatives produced by the mangrove endophytic fungus No. 2240. Chem. Nat. Compd. 2008, 44, 296–300. [Google Scholar] [CrossRef]
- Li, D.M.; Wu, X.; Ji, X.C.; Wu, X.Y.; Bai, J.; Pei, Y.H. Secondary metabolites of endophyte fungus Alternaria tenuissima SY-P-07. Chin. Pharm. J. 2014, 49, 464–468. [Google Scholar]
- Zhang, S.Y.; Li, Z.L.; Bai, J.; Wang, Y.; Zhang, L.M.; Wu, X.; Hua, M.H. A new perylenequinone from a halotolerant fungus, Alternaria sp. M6. Chin. J. Nat. Med. 2012, 10, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Stack, M.E.; Mazzola, E.P.; Page, S.W.; Pohland, A.E.; Highet, R.J. Mutagenic perylenequinone metabolites of Alternaria alternata: Altertoxins I, II, and III. J. Nat. Prod. 1986, 52, 866–871. [Google Scholar] [CrossRef]
- Sun, J.Y.; Awakawa, T.; Noguchi, H.; Abe, I. Induced production of mycotoxins in an endophytic fungus from the medicinal plant Datura stramonium L. Bioorg. Med. Chem. Lett. 2012, 22, 6397–6400. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Fu, X.M.; Zhang, X.L.; Kong, W.W.; Wang, C.Y. Bioactive perylene derivatives from a soft coral-derived fungus Alternaria sp. (ZJ-2008017). Chem. Nat. Compd. 2015, 51, 766–768. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Yang, Q.; Xia, G.P.; Huang, H.B.; Li, H.X.; Ma, L.; Lu, Y.J.; He, L.; Xia, X.K.; She, Z.G. Polyketides with α-glucosidase inhibitory activity from a mangrove endophytic fungus, Penicillium sp. HN29-3B1. J. Nat. Prod. 2015, 78, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds of 1–13 are available from the authors. |

 ) and HMBC (
) and HMBC (  ) correlations for compounds 1, 2.
) correlations for compounds 1, 2.
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 171.2, C | 200.7, C | ||
| 2 | 73.8, C | |||
| 3 | 5.82 (dd, 8.0, 4.6) | 78.4, CH | 200.8, C | |
| 3a | 152.2, C | 143.4, C | ||
| 4 | 7.00 (d, 7.4) | 113.9, CH | 7.44 (d, 7.4) | 115.5, CH |
| 5 | 7.55 (dd, 8.2, 7.4) | 137.8, CH | 7.77 (dd, 8.2, 7.4) | 139.1, CH |
| 6 | 6.90 (d, 8.2) | 117.1, CH | 7.28 (d, 8.2) | 124.4, CH |
| 7 | 158.4, C | 158.3, C | ||
| 7a | 112.5, C | 127.4, C | ||
| 8 | 3.08 (dd, 16.6, 4.6) | 40.0, CH2 | 3.37 (d, 2.5) | 49.5, CH2 |
| 2.80 (dd, 16.6, 8.0) | ||||
| 9 | 171.5, C | 208.1, C | ||
| 10 | 3.72 (s) | 52.4, CH3 | 2.09 (s) | 29.3, CH3 |
| Compounds | IC50 (μM) | |||
|---|---|---|---|---|
| MCF-7 | HepG-2 | NCI-H460 | SF-268 | |
| 1–8 | ≥100 | ≥100 | ≥100 | ≥100 |
| 9 | 35.73 ± 1.61 | 52.38 ± 2.46 | 43.31 ± 1.75 | 49.04 ± 1.84 |
| 10 | ≥100 | ≥100 | ≥100 | ≥100 |
| 11 | 3.73 ± 0.33 | 5.30 ± 0.95 | 5.47 ± 0.26 | 6.57 ± 0.35 |
| 12 | 1.91 ± 0.17 | 5.63 ± 0.10 | 9.67 ± 0.22 | 4.25 ± 0.01 |
| 13 | ≥100 | ≥100 | ≥100 | ≥100 |
| Cisplatin | 3.09 ± 0.27 | 1.39 ± 0.18 | 2.43 ± 0.15 | 2.37 ± 0.35 |
| Compounds | α-glucosidase (IC50, μM) |
|---|---|
| 4 | 34.88 ± 1.59 |
| 5 | 102.34 ± 2.45 |
| 9 | 141.43 ± 7.66 |
| 10 | 74.94 ± 2.70 |
| 12 | 12.05 ± 2.06 |
| 13 | 166.13 ± 2.81 |
| Acarbose | 427.34 ± 12.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, H.-X.; Chen, Y.-C.; Sun, Z.-H.; Li, H.-H.; Li, S.-N.; Yan, M.-L.; Zhang, W.-M. Two New Metabolites from the Endophytic Fungus Alternaria sp. A744 Derived from Morinda officinalis. Molecules 2017, 22, 765. https://doi.org/10.3390/molecules22050765
Wang Y, Liu H-X, Chen Y-C, Sun Z-H, Li H-H, Li S-N, Yan M-L, Zhang W-M. Two New Metabolites from the Endophytic Fungus Alternaria sp. A744 Derived from Morinda officinalis. Molecules. 2017; 22(5):765. https://doi.org/10.3390/molecules22050765
Chicago/Turabian StyleWang, Ying, Hong-Xin Liu, Yu-Chan Chen, Zhang-Hua Sun, Hao-Hua Li, Sai-Ni Li, Ming-Li Yan, and Wei-Min Zhang. 2017. "Two New Metabolites from the Endophytic Fungus Alternaria sp. A744 Derived from Morinda officinalis" Molecules 22, no. 5: 765. https://doi.org/10.3390/molecules22050765
APA StyleWang, Y., Liu, H. -X., Chen, Y. -C., Sun, Z. -H., Li, H. -H., Li, S. -N., Yan, M. -L., & Zhang, W. -M. (2017). Two New Metabolites from the Endophytic Fungus Alternaria sp. A744 Derived from Morinda officinalis. Molecules, 22(5), 765. https://doi.org/10.3390/molecules22050765