One-Flask Synthesis of Pyrazolo[3,4-d]pyrimidines from 5-Aminopyrazoles and Mechanistic Study
Abstract
:1. Introduction
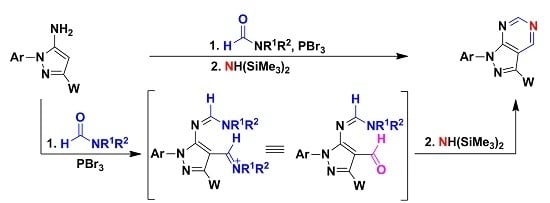
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Standard Procedure for the Synthesis of Pyrazolo[3,4-d]pyrimidines 3a–n
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ugi, I.; Dömling, A.; Werner, B. Since 1995 the new chemistry of multicomponent reactions and their libraries, including their heterocyclic chemistry. J. Heterocycl. Chem. 2000, 37, 647–658. [Google Scholar] [CrossRef]
- Posner, G.H. Multicomponent one-pot annulations forming 3 to 6 bonds. Chem. Rev. 1986, 86, 831–844. [Google Scholar] [CrossRef]
- Weber, L.; Illgen, K.; Almstetter, M. Discovery of new multi component reactions with combinatorial methods. Synlett 1999, 366–374. [Google Scholar] [CrossRef]
- Fogg, D.E.; dos Santos, E.N. Tandem catalysis: A taxonomy and illustrative review. Coord. Chem. Rev. 2004, 248, 2365–2379. [Google Scholar] [CrossRef]
- Shindoh, N.; Takemoto, Y.; Takasu, K. Auto-Tandem Catalysis: A Single Catalyst Activating Mechanistically Distinct Reactions in a Single Reactor. Chem. Eur. J. 2009, 15, 12168–12179. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.D.; Zhou, B.H.; Meng, X.G.; Wu, A.X.; Pan, Y.J. Efficient C−C Double-Bond Formation Reaction via a New Synthetic Strategy: A Self-Sorting Tandem Reaction. Org. Lett. 2006, 8, 2245–2248. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yang, Y.; Wu, Y.D.; Cong, C.; Shu, W.M.; Zhang, D.X.; Cao, L.P.; She, N.F.; Wu, A.X. An efficient synthesis of hydantoins via sustainable integration of coupled domino processes. Org. Lett. 2010, 12, 4026–4029. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Gao, Q.H.; Lian, M.; Yuan, J.J.; Liu, M.C.; Zhao, Q.; Yang, Y.; Wu, A.X. A sustainable byproduct catalyzed domino strategy: Facile synthesis of a-formyloxy and acetoxy ketones via iodination/nucleophilic substitution/hydrolyzation/oxidation sequences. Chem. Commun. 2011, 47, 12700–12702. [Google Scholar] [CrossRef] [PubMed]
- Allenn, A.E.; MacMillan, D.W.C. Synergistic catalysis: A powerful synthetic strategy for new reaction development. Chem. Sci. 2012, 3, 633–658. [Google Scholar] [CrossRef] [PubMed]
- Harschneck, T.; Kirsch, S.F. One-pot synthesis of 1, 2-Dihydropyridines: Expanding the diverse reactivity of propargyl vinyl ethers. J. Org. Chem. 2011, 76, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Jia, F.C.; Liu, M.C.; Wu, A.X. A multipathway coupled domino strategy: Metal-free oxidative cyclization for one-pot synthesis of 2-acylbenzothiazoles from multiform substrates. Org. Lett. 2012, 14, 4414–4417. [Google Scholar] [CrossRef] [PubMed]
- Guilarte, V.; Fernández-Rodríguez, M.A.; Garcría-Garcría, P.; Hernando, E.; Sanz, R. A Practical, One-Pot Synthesis of Highly Substituted Thiophenes and Benzo[b]thiophenes from Bromoenynes and o-Alkynylbromobenzenes. Org. Lett. 2011, 13, 5100–5103. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Tsai, H.J.; Huang, Y.Y.; Lin, H.Y.; Wang, L.Y.; Wu, T.S.; Wong, F.F. Selective synthesis of pyrazolo[3,4-d]pyrimidine, N-(1H-pyrazol-5-yl)formamide, or N-(1H-pyrazol-5-yl)formamidine derivatives from N-1-substituted-5-aminopyrazoles with new Vilsmeier-type reagents. Tetrahedron 2013, 69, 1378–1386. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wang, L.Y.; Chang, C.H.; Kuo, Y.H.; Kaneko, K.; Takayama, H.; Kimura, M.; Juang, S.H.; Wong, F.F. One-pot synthesis and antiproliferative evaluation of pyrazolo[3, 4-d]pyrimidine derivatives. Tetrahedron 2012, 68, 9658–9664. [Google Scholar] [CrossRef]
- Ismail, N.S.M.; Ali, E.M.H.; Ibrahim, D.A.; Serya, R.A.T.; El Ella, D.A.A. Pyrazolo[3, 4-d]pyrimidine based scaffold derivatives targeting kinases as anticancer agents. Future J. Pharm. Sci. 2016, 2, 20–30. [Google Scholar] [CrossRef]
- Peter Bonn, P.; Mikael Brink, D.; Fagerhag, J.; Jurva, U.; Robb, G.R.; Schnecke, V.; Henriksson, A.S.; Waring, M.J.; Westerlund, C. The discovery of a novel series of glucokinase activators based on a pyrazolopyrimidine scaffold. Bioorg. Med. Chem. Lett. 2012, 22, 7302–7305. [Google Scholar] [CrossRef] [PubMed]
- Bakavoli, M.; Bagherzadeh, G.; Vaseghifar, M.; Shiri, A.; Mehdi Pordel, M.; Mashreghi, M.; Pordeli, P.; Araghi, M. Molecular iodine promoted synthesis of new pyrazolo[3,4-d]pyrimidine derivatives as potential antibacterial agents. Eur. J. Med. Chem. 2010, 45, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Mahalinga, M.; Karthikeyan, M.S.; Akberali, P.M.; Shetty, N.S. Synthesis of some novel pyrazole[3,4-d]pyrimidine derivatives as potential antimicrobial agents. Bioorg. Med. Chem. 2006, 14, 2040–2047. [Google Scholar] [CrossRef] [PubMed]
- Shamroukh, A.H.; Rashad, A.E.; Sayed, H.H. Synthesis of Some Pyrazolo[3,4-d]pyrimidine Derivatives for Biological Evaluation. Phosphorous Sulfur Silicon Relat. Elem. 2005, 180, 2347–2360. [Google Scholar] [CrossRef]
- Rigueiroa, F.; Teixeiraa, S.; Salaheldinb, A.M.; Oliveira-Camposb, A.M.F.; Rodriguesb, L.M.; Peixotoc, F.; Oliveira, M.M. Evaluation of antioxidant properties of some pyrazolo[3,4-d]pyrimidines derivatives and their effects on mitochondria bioenergetics. Biochim. Biophys. Acta 2008, 1777, S10.17. [Google Scholar] [CrossRef]
- Kumar, A.; Ahmad, I.; Chhikara, B.S.; Tiwari, R.; Mandal, D.; Parang, K. Synthesis of 3-phenylpyrazolopyrimidine-1,2,3-triazole conjugates and evaluation of their Src kinase inhibitory and anticancer activities. Bioorg. Med. Chem. Lett. 2011, 21, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Ragab, F.A.; Alqasoumi, S.I.; Alafeefy, A.M.; Aboulmag, S.A. Synthesis of some new pyrazolo[3,4-d]pyrimidine derivatives of expected anticancer and radioprotective activity. Eur. J. Med. Chem. 2010, 45, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Schenone, S.; Bruno, O.; Bondavalli, F.; Ranise, A.; Mosti, L.; Giulia Menozzi, G.; Paola Fossa, P.; Donnini, S.; Santoro, A.; Ziche, M.; et al. Antiproliferative activity of new 1-aryl-4-amino-1H-pyrazolo[3,4-d]pyrimidine derivatives toward the human epidermoid carcinoma A431 cell line. Eur. J. Med. Chem. 2004, 39, 939–946. [Google Scholar] [CrossRef] [PubMed]
- EI-Bendary, E.R.; Badria, F.A. Synthesis, DNA-binding, and Antiviral Activity of Certain Pyrazolo[3,4-d]pyrimidine Derivatives. Arch. Pharm. 2000, 333, 99–103. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.Q.; Liu, Z.J. Synthesis and herbicidal activity of novel pyrazolo[3,4-d]pyrimidin-4-one derivatives containing aryloxyphenoxypropionate moieties. Bioorg. Med. Chem. Lett. 2007, 17, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Chern, J.H.; Shia, K.S.; Hsu, T.A.; Tai, C.L.; Lee, C.C.; Lee, Y.C.; Chang, C.S.; Tseng, S.N.; Shih, S.R. Design, synthesis, and structure–activity relationships of pyrazolo [3,4-d] pyrimidines: A novel class of potent enterovirus inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 2519–2525. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.E.; Hegab, M.I.; Abdel-Megeid, R.E.; Fathalla, N.; Abdel-Megeid, F.M.E. Synthesis and anti-HSV-1 evaluation of some pyrazoles and fused pyrazolopyrimidines. Eur. J. Med. Chem. 2009, 44, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Rahmoui, A.; Souiei, S.; Belkacem, M.A.; Romdhane, A.; Bouajila, J.; Jannet, H.B. Synthesis and biological evaluation of novel pyrazolopyrimidines derivatives as anticancer and anti-5-lipoxygenase agents. Bioorg. Chem. 2016, 66, 160–168. [Google Scholar] [CrossRef] [PubMed]
- El-Kalyoubi, S.; Agili, F. A Novel Synthesis of Fused Uracils: Indenopyrimidopyridazines, Pyrimidopyridazines, and Pyrazolopyrimidines for Antimicrobial and Antitumor Evalution. Molecules 2016, 21, 1714. [Google Scholar] [CrossRef] [PubMed]
- Quintela, J.M.; Peinador, C.; Gonzalez, L.; Devesa, I.; Ferrandiz, M.L.; Alcaraz, M.J.; Riguera, R. 6-Dimethylamino 1H-pyrazolo[3,4-d]pyrimidine derivatives as new inhibitors of inflammatory mediators in intact cell. Bioorg. Med. Chem. 2003, 11, 863–868. [Google Scholar] [CrossRef]
- Abdelazeem, A.H.; Abdelatef, S.A.; El-Saadi, M.T.; Omar, H.A.; Khan, S.I.; McCurdy, C.R.; El-Moghazy, S.M. Novel pyrazolopyrimidine derivatives targeting COXs and iNOS enzymes; design, synthesis and biological evaluation as potential anti-inflammatory agents. Eur. J. Pharm. Sci. 2014, 62, 197–221. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, K.; Farooq, S.M.; Raghunandan, R.; Ma, P.R. Design and synthesis of pyrazolo[3,4-d]pyrimidine core based dissymmetrical ‘Leonard linker’ compounds: 1H-NMR and crystallographic evidence for folded conformation due to arene interaction. J. Mol. Struct. 2006, 785, 106–113. [Google Scholar] [CrossRef]
- Venkatesan, G.; Paira, P.; Cheong, L.C.; Vamsikrishna, K.; Federico, S.; Klotz, K.N.; Spalluto, G.; Pastorin, G. Discovery of simplified N2-substituted pyrazolo[3,4-d]pyrimidine derivatives as novel adenosine receptor antagonists: Efficient synthetic approaches, biological evaluations and molecular docking studies. Bioorg. Med. Chem. 2014, 22, 1751–1765. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.; Arques, A.; Vinader, M.V. Tandem aza-wittig reaction/electrocyclic ring-closure a facile entry to the synthesis of fused pyrimidines: Preparation of pyrazolo[3,4-d] and 1,2,3-triazolo[4,5-d]pyrimidine derivatives. Tetrahedron Lett. 1987, 28, 4451–4454. [Google Scholar] [CrossRef]
- Sureja, D.K.; Dholakia, S.P.; Vadalia, K.R. Synthesis of some novel pyrazolo[3,4-d] pyrimidin-4(5H)-one derivatives as potential antimicrobial agents. J. Pharm. Bioallied. Sci. 2016, 8, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Slavish, P.J.; Price, J.E.; Hanumesh, P.; Webb, T.R. Efficient synthesis of pyrazolopyrimidine libraries. J. Comb. Chem. 2010, 12, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.M.; Caltabiano, S.; Koehn, F.E.; Chen, Z.J.; Francisco, G.D.; Ellingboe, J.W.; Kharode, Y.; Mangine, A.; Francis, R.; TrailSmith, M.; et al. Pyrazolopyrimidine-2,4-dione sulfonamides: Novel and selective calcitonin inducers. J. Med. Chem. 2002, 45, 2342–2345. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.S.; Lin, H.Y.; Huang, Y.Y.; Kaneko, K.; Takayama, H.; Kimura, M.; Juang, S.H.; Wong, F.F. Chemoselective synthesis, antiproliferative activities, and SAR study of 1H-pyrazol-5-yl-N,N-dimethyl-formamidines and pyrazolyl-2-azadienes. Med. Chem. Res. 2012, 21, 3920–3928. [Google Scholar] [CrossRef]
- Cheng, K.M.; Huang, Y.Y.; Huang, J.J.; Kaneko, K.; Kimura, M.; Takayama, H.; Juang, S.H.; Wong, F.F. Synthesis and antiproliferative evaluation of N,N-disubstituted-N′-[1-aryl-1H-pyrazol-5-yl]-methnimidamides. Bioorg. Med. Chem. Lett. 2010, 20, 6781–6784. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Negi, A.S.; Kumar, J.K.; Gupta, M.M. A simple, convenient and chemoselective formylation of sterols by Vilsmeier reagent. Steroids 2006, 71, 632–638. [Google Scholar] [CrossRef] [PubMed]
- El-Shishtawy, R.M.; Almeida, P. A new Vilsmeier-type reaction for one-pot synthesis of pH sensitive fluorescent cyanine dyes. Tetrahedron 2006, 62, 7793–7798. [Google Scholar] [CrossRef]
- Pan, W.; Dong, D.; Wang, K.; Zhang, J.; Wu, R.; Xiang, D.; Liu, Q. Efficient One-Pot Synthesis of Highly Substituted Pyridin-2(1H)-ones via the Vilsmeier−Haack Reaction of 1-Acetyl,1-Carbamoyl Cyclopropanes. Org. Lett. 2007, 9, 2421. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Kaneko, K.; Takayama, H.; Kimura, M.; Wong, F.F. New investigation of Vilsmeier-type reaction using pyrazolones with various amides. Tetrahedron Letts. 2011, 52, 3786–3792. [Google Scholar] [CrossRef]
- Wong, F.F.; Huang, Y.Y. Novel Vilsmeier-type methylenation for synthesis of dipyrazolylmethane derivatives using formamide or N-methylformamide. Tetrahedron 2011, 67, 3863–3867. [Google Scholar] [CrossRef]
- Simay, A.; Takacs, K.; Horvath, K.; Dvortsak, P. Vilsmeier-Haack reaction of 5-amino- and 5-acylaminopyrazoles. Acta Chim. Acad. Sci. Hung. 1980, 105, 127–139. [Google Scholar]
- Jachak, M.N.; Avhale, A.B.; Medhane, V.J.; Toche, R.B. A convenient route for the synthesis of pyrazolo[3,4-d]pyrimidine, pyrazolo[3,4-b][1,6]naphthyridine and pyrazolo[3,4-b]quinoline derivatives. J. Heterocycl. Chem. 2006, 43, 1169–1175. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Entry | Amine Agents | Equiv. | Yields (%) of Compound 3a |
|---|---|---|---|
| 1 | Without base | - | - a |
| 2 | Hexamethylenetetramine | 3 | 33 |
| 3 | Lithium bis(trimethylsilyl)amine (LiN(SiMe3)2) | 3 | 67 |
| 4 | Sodium bis(trimethylsilyl)amine (NaN(SiMe3)2) | 3 | 81 |
| 5 | Hexamethyldisilazane (NH(SiMe3)2) | 3 | 91 |
| 6 | Hexamethyldisilazane (NH(SiMe3)2) | 1 | 56 |
| 7 | Hexamethyldisilazane (NH(SiMe3)2) | 2 | 63 |
| 8 | Hexamethyldisilazane (NH(SiMe3)2) | 4 | 75 |

| Entry | Amide Solvents | Yields (%) of Compound 3a |
|---|---|---|
| 1 | N,N-dimethylformamide (DMF) | 91 |
| 2 | N,N-diethylformamide (DEF) | 86 |
| 3 | N,N-diisopropylformamide | 83 |
| 4 | N,N-di-n-butylformamide | 81 |
| 5 | piperidine-1-carbaldehyde | 69 |
| 6 | pyrrolidine-1-carbaldehyde | 56 |

| Substrates | X | W | No. | Yields of 3a–n (%) | |
|---|---|---|---|---|---|
| Intermolecular Reaction | Intramolecular Reaction a | ||||
| 1a | Ph | Ph | 3a | 91 | 96 |
| 1b | o-Me-Ph | Ph | 3b | 78 | 93 |
| 1c | o-Cl-Ph | Ph | 3c | 86 | 91 |
| 1d | m-Me-Ph | Ph | 3d | 89 | 92 |
| 1e | m-Cl-Ph | Ph | 3e | 91 | 92 |
| 1f | m-NO2-Ph | Ph | 3f | 87 | 87 |
| 1g | p-Me-Ph | Ph | 3g | 91 | - |
| 1h | p-Cl-Ph | Ph | 3h | 87 | 91 |
| 1i | p-Br-Ph | Ph | 3i | 81 | 95 |
| 1j | Ph | Me | 3j | 79 | 93 |
| 1k | Ph | t-Bu | 3k | 87 | 91 |
| 1l | Ph | p-Me-Ph | 3l | 84 | 93 |
| 1m | Ph | p-Cl-Ph | 3m | 88 | 91 |
| 1n | Ph | p-OMe-Ph | 3n | 91 | 94 |

| Entry | Substrates | NR1R2 | Amines | Yields of 3a (%) | |
|---|---|---|---|---|---|
| 1 | 7a | NMe2 | Hexamethylenetetramine | 3a | 37 |
| 2 | 7a | NMe2 | LiN(SiMe3)2 | 3a | 51 |
| 3 | 7a | NMe2 | NaN(SiMe3)2 | 3a | 84 |
| 4 | 7a | NMe2 | NH(SiMe3)2 | 3a | 91 |
| 5 | 7b | NEt2 | NH(SiMe3)2 | 3a | 89 |
| 6 | 7c | N(i-Pr)2 | NH(SiMe3)2 | 3a | 61 |
| 7 | 7d | N(n-Bu)2 | NH(SiMe3)2 | 3a | 81 |
| 8 | 7e | Piperidinyl | NH(SiMe3)2 | 3a | 86 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, W.-P.; Tsai, S.-E.; Uramaru, N.; Takayama, H.; Wong, F.F. One-Flask Synthesis of Pyrazolo[3,4-d]pyrimidines from 5-Aminopyrazoles and Mechanistic Study. Molecules 2017, 22, 820. https://doi.org/10.3390/molecules22050820
Yen W-P, Tsai S-E, Uramaru N, Takayama H, Wong FF. One-Flask Synthesis of Pyrazolo[3,4-d]pyrimidines from 5-Aminopyrazoles and Mechanistic Study. Molecules. 2017; 22(5):820. https://doi.org/10.3390/molecules22050820
Chicago/Turabian StyleYen, Wan-Ping, Shuo-En Tsai, Naoto Uramaru, Hiroyuki Takayama, and Fung Fuh Wong. 2017. "One-Flask Synthesis of Pyrazolo[3,4-d]pyrimidines from 5-Aminopyrazoles and Mechanistic Study" Molecules 22, no. 5: 820. https://doi.org/10.3390/molecules22050820
APA StyleYen, W. -P., Tsai, S. -E., Uramaru, N., Takayama, H., & Wong, F. F. (2017). One-Flask Synthesis of Pyrazolo[3,4-d]pyrimidines from 5-Aminopyrazoles and Mechanistic Study. Molecules, 22(5), 820. https://doi.org/10.3390/molecules22050820