Structural Characterization of a Rhamnogalacturonan I Domain from Ginseng and Its Inhibitory Effect on Galectin-3
Abstract
:1. Introduction
2. Results and Discussion
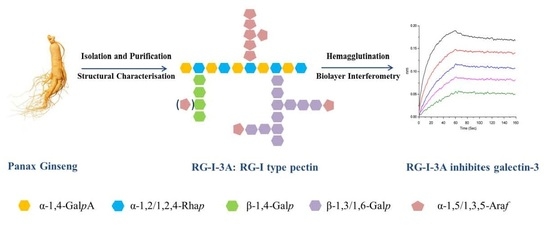
2.1. Preparation of RG-I-3A from Ginseng Polysaccharides
2.2. Partial Acid Hydrolysis of RG-I-3A
2.3. Monoclonal Antibody Detection of RG-I-3A Hydrolysis Products
2.4. NMR Spectra Analysis of RG-I-3A Hydrolysis Products
2.5. Inhibitory Effect of RG-I-3A on Galectin-3
3. Materials and Methods
3.1. Materials
3.2. Preparation of RG-I-3A from Ginseng Polysaccharides
3.3. Molecular Weight Distribution and Monosaccharide Composition Analysis
3.4. Partial Acid Hydrolysis
3.5. ELISA Assay
3.6. NMR Spectra Analysis
3.7. Galectin-3-Mediated Hemagglutination Assay
3.8. Biolayer Interferometry Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Paniagua, C.; Kirby, A.R.; Gunning, A.P.; Morris, V.J.; Matas, A.J.; Quesada, M.A.; Mercado, J.A. Unravelling the nanostructure of strawberry fruit pectins by endo-polygalacturonase digestion and atomic force microscopy. Food Chem. 2017, 224, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Ralet, M.C.; Thibault, J.F. Interchain heterogeneity of enzymatically deesterified lime pectins. Biomacromolecules 2002, 3, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.T.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant. Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Vincken, J.P.; Schols, H.A.; Oomen, R.J.; McCann, M.C.; Ulvskov, P.; Voragen, A.G.; Visser, R.G. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol. 2003, 132, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B.M. Rhamnogalacturonan-I: A structurally puzzling and functionally versatile polysaccharide from plant cell walls and mucilages. Polym. Rev. 2011, 51, 391–413. [Google Scholar] [CrossRef]
- Leclere, L.; Cutsem, P.V.; Michiels, C. Anti-cancer activities of pH- or heat-modified pectin. Front. Pharmacol. 2013, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.H.; Park, J.H. Cyclooxygenase inhibitory activity of ginsenosides from heat-processed ginseng. Food Chem. 2012, 133, 998–1000. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Bi, H.T.; Li, X.H.; Ni, W.H.; Han, H.; Li, N.; Wang, B.Q.; Zhou, Y.F.; Tai, G.H. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng C.A. Meyer. Carbohyd. Polym. 2009, 77, 544–552. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Cheng, H.R.; Li, S.S.; Wang, J.; Liu, D.; Hao, M.; Gao, X.G.; Fan, E.X.; Tai, G.H.; Zhou, Y.F. Relationship of the inhibition of cell migration with the structure of ginseng pectic polysaccharides. Carbohyd. Polym. 2010, 81, 340–347. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Li, S.S.; Liu, X.Y.; Sun, L.; Liu, H.B.; Iteku, J.; Zhou, Y.F.; Tai, G.H. Rhamnogalacturonan I domains from ginseng pectin. Carbohyd. Polym. 2010, 79, 811–817. [Google Scholar] [CrossRef]
- Cheng, H.R.; Li, S.S.; Fan, Y.Y.; Gao, X.G.; Hao, M.; Wang, J.; Zhang, X.Y.; Tai, G.H.; Zhou, Y.F. Comparative studies of the antiproliferative effects of ginseng polysaccharides on HT-29 human colon cancer cells. Med. Oncol. 2011, 28, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.S.; Fan, Y.Y.; Chen, Y.; Liu, D.; Cheng, H.R.; Gao, X.G.; Zhou, Y.F. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C.A. Meyer. J. Ethnopharmacol 2010, 130, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Flaisher-Grinberg, S.; Li, S.S.; Liu, H.B.; Sun, L.; Zhou, Y.F.; Einat, H. Antidepressant-like effects of the active acidic polysaccharide portion of ginseng in mice. J. Ethnopharmacol 2010, 132, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.H.; Zhang, X.; Wang, B.; Chen, Y.; Han, H.; Fan, Y.Y.; Zhou, Y.F.; Tai, G.H. Antitumor activities and immunomodulatory effects of ginseng neutral polysaccharides in combination with 5-fluorouracil. J. Med. Food 2010, 13, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta. 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Iurisci, I.; Tinari, N.; Natoli, C.; Angelucci, D.; Cianchetti, E.; Iacobelli, S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin. Cancer Res. 2000, 6, 1389–1393. [Google Scholar] [PubMed]
- Gao, X.G.; Zhi, Y.; Sun, L.; Peng, X.X.; Zhang, T.; Xue, H.T.; Tai, G.H.; Zhou, Y.F. The inhibitory effects of an RG-I domain from ginseng pectin on galectin-3 and its structure-activity relationship. J. Biol. Chem. 2013, 288, 33953–33965. [Google Scholar] [CrossRef] [PubMed]
- Ralet, M.C.; Crépeau, M.J.; Lefèbvre, J.; Mouille, G.; Hofte, H.; Thibault, J.F. Reduced number of homogalacturonan domains in pectins of an Arabidopsis mutant enhances the flexibility of the polymer. Biomacromolecules 2008, 9, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohyd. Res. 2009, 344, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Seymour, G.B.; Knox, J.P. Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-d-galactan. Plant. Physiol. 1997, 113, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.T.; Marcus, S.E.; Knox, J.P. Generation of a monoclonal antibody specific to (1→5)-α-l-arabinan. Carbohyd. Res. 1998, 308, 149–152. [Google Scholar] [CrossRef]
- Moller, I.; Marcus, S.E.; Haeger, A.; Verhertbruggen, Y.; Verhoef, R.; Schols, H.A.; Ulvskov, P.; Mikkelsen, J.D.; Knox, J.P.; Willats, W. High-throughput screening of monoclonal antibodies against plant cell wall glycans by hierarchical clustering of their carbohydrate microarray binding profiles. Glycoconjugate. J. 2008, 25, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.P.; Linstead, P.J.; Peart, J.; Cooper, C.; Roberts, K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant. J. 1991, 1, 317–326. [Google Scholar]
- Samuelsen, A.B.; Westereng, B.; Yousif, O.; Holtekjolen, A.K.; Michaelsen, T.E.; Knutsen, S.H. Structural features and complement-fixing activity of pectin from three Brassica oleracea varieties: White cabbage, kale, and red kale. Biomacromolecules 2007, 8, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Chen, X. Structure elucidation and immunological activity of a novel pectic polysaccharide from the stems of Avicennia marina. Eur. Food Res. Technol. 2013, 236, 243–248. [Google Scholar] [CrossRef]
- Gur'janov, O.P.; Gorshkova, T.A.; Kabel, M.; Schols, H.A.; van Dam, J.E.G. MALDI-TOF MS evidence for the linking of flax bast fibre galactan to rhamnogalacturonan backbone. Carbohyd. Polym. 2007, 67, 86–96. [Google Scholar] [CrossRef]
- Lin, L.Y.; Wang, P.P.; Du, Z.Y.; Wang, W.C.; Cong, Q.F.; Zheng, C.P.; Jin, C.; Ding, K.; Shao, C.H. Structural elucidation of a pectin from flowers of Lonicera japonica and its antipancreatic cancer activity. Int. J. Biol. Macromol. 2016, 88, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Sathisha, U.V.; Jayaram, S.; Harish Nayaka, M.A.; Dharmesh, S.M. Inhibition of galectin-3 mediated cellular interactions by pectic polysaccharides from dietary sources. Glycoconj. J. 2007, 24, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zheng, Y.; Zhao, D.Y.; Yan, J.M.; Sun, C.L.; Zhou, Y.F.; Tai, G.H. Multiple approaches to assess pectin binding to galectin-3. Int. J. Biol. Macromol. 2016, 91, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, Y.F.; Knox, J.P. Ginseng root water-extracted pectic polysaccharides originate from secretory cavities. Planta. 2011, 234, 487–499. [Google Scholar] [CrossRef] [PubMed]





| Fraction | Mw (kDa) | Sugar Compositions (mol %) | ||||||
|---|---|---|---|---|---|---|---|---|
| GalA | Rha | Gal | Ara | GlcA | Glc | Man | ||
| RG-I-3A | 50.0 ± 1.05 | 32.5±0.76 | 11.2 ± 0.38 | 31.9 ± 0.66 | 16.5 ± 0.35 | 3.0 ± 0.15 | 1.9 ± 0.30 | 2.1 ± 0.22 |
| RG-I-3A-0.5 | 43.0 ± 0.96 | 33.2 ± 0.55 | 30.3 ± 0.47 | 21.6 ± 0.54 | 8.0 ± 0.30 | 2.1 ± 0.21 | 2.6 ± 0.26 | 1.6 ± 0.25 |
| RG-I-3A-2 | 21.5 ± 0.88 | 37.8 ± 0.45 | 37.2 ± 0.45 | 20.3 ± 0.50 | --- | 2.4 ± 0.17 | 1.7 ± 0.19 | 1.7 ± 0.20 |
| RG-I-3A-4 | 7.2 ± 0.80 | 40.2 ± 0.48 | 39.6 ± 0.51 | 14.8 ± 0.45 | --- | 2.5 ± 0.17 | 1.9 ± 0.15 | 0.9 ± 0.15 |
| RG-I-3A-6 | 6.3 ± 0.55 | 41.2 ± 0.52 | 40.1 ± 0.36 | 13.3 ± 0.37 | --- | 2.9 ± 0.22 | 2.2 ± 0.23 | 0.3 ± 0.09 |
| RG-I-3A-16 | 6.0 ± 0.60 | 45.6 ± 0.61 | 44.8 ± 0.44 | 5.7 ± 0.32 | --- | 1.9 ± 0.15 | --- | 1.9 ± 0.18 |
| Pectin | Antibody | Antigen Epitope | Reference |
|---|---|---|---|
| HG a | LM19 | Partially Me-HG/de-esterified HG | [19] |
| HG a | LM20 | Partially Me-HG | [19] |
| RG-I b | LM5 | (1→4)-β-D-galactan (~ four (1→4)-β-Gal) | [20] |
| RG-I b | LM6 | Linear (1→5)-α-L-arabinan (~five (1→5)-α-Ara) | [21] |
| AGP c | LM14 | AGP glycan (AG-II) | [22] |
| AGP c | JIM13 | AGP glycan (AG-II) | [23] |
| AGP c | JIM16 | AGP glycan (AG-II) | [23] |
| Fraction | Sugar Residues | Chemical Shifts, δ (ppm) | |||||
|---|---|---|---|---|---|---|---|
| C-1/H-1 | C-2/H-2 | C-3/H-3 | C-4/H-4 | C-5/H-5 | C-6/H-6 | ||
| RG-I-3A | →4)-α-GalpA-(1→ | 97.51/5.02 | 66.78/3.87 | 68.26/3.83 | 76.30/4.34 | 70.05/4.94 | 172.44/-- |
| →2)-α-Rhap-(1→ | 96.44/5.17 | 75.63/4.06 | 69.05/3.77 | 69.65/3.33 | 67.57/3.53 | 15.50/1.18 | |
| →2,4)-α-Rhap-(1→ | 96.44/5.17 | 75.63/4.06 | 69.05/3.75 | 76.10/3.54 | 67.57/3.55 | 15.72/1.24 | |
| t-β-Galp-(1→ | 102.26/4.54 | 70.84/3.45 | 73.39/3.61 | 67.99/4.09 | 74.59/3.65 | 59.85/3.73 | |
| →4)-β-Galp-(1→ | 102.59/4.54 | 71.72/3.45 | 72.73/3.61 | 75.47/4.14 | 73.39/3.70 | 59.85/3.73 | |
| →3)-β-Galp-(1→ | 103.22/4.57 | 71.74/3.45 | 79.36/-- | 67.99/4.09 | 74.59/3.65 | 59.94/3.73 | |
| →6)-β-Galp-(1→ | 103.22/4.55 | 71.74/3.45 | 73.39/3.61 | 67.99/4.09 | 74.59/3.66 | 67.17/4.14 | |
| →3,6)-β-Galp-(1→ | 102.37/4.57 | 71.74/3.45 | 79.36/-- | 67.99/4.09 | 74.59/3.66 | 67.17/4.14 | |
| →5)-α-Araf-(1→ | 106.45/5.05 | 81.06/4.22 | 79.75/-- | 81.67/4.14 | 65.83/3.88 | --/-- | |
| →3,5)-α-Araf-(1→ | 106.36/5.05 | 79.85/4.22 | 82.90/-- | 80.13/4.14 | 65.12/3.88 | --/-- | |
| t-α-Araf-(1→ | 108.26/5.08 | 81.06/4.22 | 79.75/-- | 81.27/4.14 | 60.13/3.61 | --/-- | |
| RG-I-3A-0.5 | →4)-α-GalpA-(1→ | 97.96/4.96 | 66.50/3.84 | 68.96/3.83 | 76.66/4.38 | 70.47/4.93 | 171.45/-- |
| →2)-α-Rhap-(1→ | 96.61/5.14 | 75.30/4.09 | 69.62/3.79 | 70.13/3.36 | 68.24/3.58 | 15.30/1.18 | |
| →2,4)-α-Rhap-(1→ | 96.86/5.14 | 75.30/4.09 | 69.61/3.78 | 76.05/3.54 | 68.24/3.59 | 15.54/1.25 | |
| t-β-Galp-(1→ | 102.28/4.54 | 70.25/3.46 | 73.28/3.65 | 67.06/4.00 | 73.35/3.68 | 59.76/3.78 | |
| →6)-β-Galp-(1→ | 103.12/4.55 | 69.86/3.46 | 73.28/3.65 | 67.06/4.00 | 73.35/3.93 | 67.06/4.14 | |
| →3,6)-β-Galp-(1→ | 102.54/4.57 | 69.86/3.47 | 80.02/-- | 67.06/4.00 | 73.35/3.93 | 67.06/4.14 | |
| RG-I-3A-2 | →4)-α-GalpA-(1→ | 98.08/4.93 | 66.65/3.85 | 68.38/3.80 | 76.78/4.38 | 70.62/4.81 | 171.61/-- |
| →2)-α-Rhap-(1→ | 96.74/5.15 | 75.42/4.06 | 69.11/3.78 | 70.62/3.31 | 68.18/3.59 | 15.43/1.22 | |
| →2,4)-α-Rhap-(1→ | 96.99/5.15 | 75.42/4.06 | 69.11/3.78 | 76.22/3.55 | 68.18/3.61 | 15.68/1.27 | |
| t-β-Galp-(1→ | 102.41/4.53 | 70.13/3.53 | 73.75/3.66 | 67.54/4.00 | 73.42/3.68 | 59.89/3.76 | |
| →6)-β-Galp-(1→ | 103.25/4.55 | 69.65/3.53 | 73.75/3.66 | 67.54/4.00 | 73.42/3.94 | 67.75/4.11 | |
| →3,6)-β-Galp-(1→ | 102.64/4.56 | 69.65/3.55 | --/-- | 67.54/4.02 | 73.42/3.94 | 67.75/4.11 | |
| RG-I-3A-16 | →4)-α-GalpA-(1→ | 98.00/4.96 | 66.65/3.81 | 68.31/3.77 | 76.70/4.38 | 70.62/4.88 | 171.86/-- |
| →2)-α-Rhap-(1→ | 96.69/5.14 | 75.37/4.06 | 68.47/3.69 | 69.24/3.31 | 68.21/3.59 | 15.44/1.22 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Yu, L.; Shi, Y.; Lu, J.; Teng, H.; Zhou, Y.; Sun, L. Structural Characterization of a Rhamnogalacturonan I Domain from Ginseng and Its Inhibitory Effect on Galectin-3. Molecules 2017, 22, 1016. https://doi.org/10.3390/molecules22061016
Shi H, Yu L, Shi Y, Lu J, Teng H, Zhou Y, Sun L. Structural Characterization of a Rhamnogalacturonan I Domain from Ginseng and Its Inhibitory Effect on Galectin-3. Molecules. 2017; 22(6):1016. https://doi.org/10.3390/molecules22061016
Chicago/Turabian StyleShi, Huimin, Li Yu, Yun Shi, Jiaojiao Lu, He Teng, Yifa Zhou, and Lin Sun. 2017. "Structural Characterization of a Rhamnogalacturonan I Domain from Ginseng and Its Inhibitory Effect on Galectin-3" Molecules 22, no. 6: 1016. https://doi.org/10.3390/molecules22061016
APA StyleShi, H., Yu, L., Shi, Y., Lu, J., Teng, H., Zhou, Y., & Sun, L. (2017). Structural Characterization of a Rhamnogalacturonan I Domain from Ginseng and Its Inhibitory Effect on Galectin-3. Molecules, 22(6), 1016. https://doi.org/10.3390/molecules22061016