Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review
Abstract
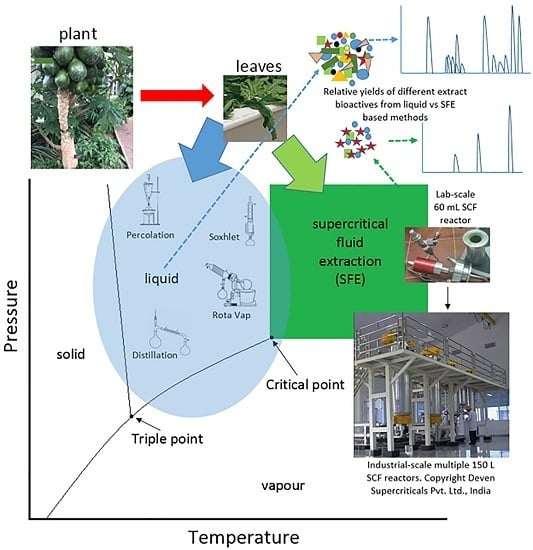
:1. Introduction
2. Sample Preparation
3. Operating Parameters
3.1. Effect of Temperature and Pressure
3.2. Effect of Organic Modifier
3.3. Effect of Water in SFE
4. Ultrasonic-Assisted Supercritical Fluid Extraction
5. Scale Up
6. Outlook of the Field
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dewick, P.M. Secondary metabolism: The building blocks and construction mechanisms. In Medicinal Natural Products; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 7–38. [Google Scholar]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Butler, M.S.; Fontaine, F.; Cooper, M.A. Natural product libraries: Assembly, maintenance, and screening. Planta Med. 2014, 80, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Luque de Castro, M.D.; Garcı́a-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Harbourne, N.; Marete, E.; Jacquier, J.C.; O′Riordan, D. Conventional extraction techniques for phytochemicals. In Handbook of Plant Food Phytochemicals; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 397–411. [Google Scholar]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefin. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Sovilj, M.N.; Nikolovski, B.G.; Spasojević, M.D. Critical review of supercritical fluid extraction of selected spice plant materials. Maced. J. Chem. Chem. Eng. 2011, 30, 197–220. [Google Scholar]
- Capuzzo, A.; Maffei, M.; Occhipinti, A. Supercritical fluid extraction of plant flavors and fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef] [PubMed]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental design of supercritical fluid extraction—A review. J. Food Eng. 2014, 124, 105–116. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Mita, G. Supercritical carbon dioxide extraction of carotenoids from pumpkin (cucurbita spp.): A review. Int. J. Mol. Sci. 2014, 15, 6725–6740. [Google Scholar] [CrossRef] [PubMed]
- King, J.W. Modern supercritical fluid technology for food applications. Ann. Rev. Food Sci. Technol. 2014, 5, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M. Supercritical fluids in separation science—The dreams, the reality and the future. J. Chromatogr. A 1999, 856, 83–115. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 k at pressures up to 800 mpa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Cui, Y.; Ang, C.Y.W. Supercritical fluid extraction and high-performance liquid chromatographic determination of phloroglucinols in st. John’s wort (Hypericum perforatum L.). J. Agric. Food Chem. 2002, 50, 2755–2759. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.C.; Medeiros-Neves, B.; Ferreira Teixeira, H.; Kawanoa, D.; Eifler-Lima, V.L.; Cassel, E.; Vargas, R.M.F.; von Poser, G.L. Supercritical CO2 extraction as a selective method for the obtainment of coumarins from pterocaulon balansae (asteraceae). J. CO2 Util. 2017, 18, 303–308. [Google Scholar] [CrossRef]
- Levy, J.M. Supercritical fluid-solid sample preparation: A selective extraction strategy. LC-GC 1999, 17, 14–21. [Google Scholar]
- Taleb, A. Supercritical fluids. In Nanomaterials and Nanochemistry; Bréchignac, C., Houdy, P., Lahmani, M., Eds.; Springer: Berlin, Germany, 2007; pp. 473–485. [Google Scholar]
- Reid R, S.T. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Gas encyclopaedia Air LiquideTM. Online Resource Operated by m-Lab (molecule-Lab). Available online: https://encyclopedia.airliquide.com/ (accessed on 14 July 2017).
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Martín, L.; Marqués, J.; González-Coloma, A.; Mainar, A.; Palavra, A.; Urieta, J. Supercritical methodologies applied to the production of biopesticides: A review. Phytochem. Rev. 2012, 11, 413–431. [Google Scholar] [CrossRef]
- Girotra, P.; Singh, S.K.; Nagpal, K. Supercritical fluid technology: A promising approach in pharmaceutical research. Pharm. Dev. Technol. 2013, 18, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Mattea, F.; Martín, Á.; Cocero, M.J. Carotenoid processing with supercritical fluids. J. Food Eng. 2009, 93, 255–265. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Gañán, N.A.; Dambolena, J.S.; Martini, R.E.; Bottini, S.B. Supercritical carbon dioxide fractionation of peppermint oil with low menthol content—Experimental study and simulation analysis for the recovery of piperitenone. J. Supercrit. Fluids 2015, 98, 1–11. [Google Scholar] [CrossRef]
- Yu, I.L.; Yu, Z.R.; Koo, M.; Wang, B.J. A continuous fractionation of ginsenosides and polysaccharides from Panax ginseng using supercritical carbon dioxide technology. J. Food Process. Preserv. 2016, 40, 743–748. [Google Scholar] [CrossRef]
- Romero De La Vega, G.; Salgado Cervantes, M.A.; Garcia Alvarado, M.A.; Romero-Martínez, A.; Hegel, P.E. Fractionation of vanilla oleoresin by supercritical CO2 technology. J. Supercrit. Fluids 2016, 108, 79–88. [Google Scholar] [CrossRef]
- Yoon, S.W.; Pyo, Y.G.; Lee, J.; Lee, J.S.; Kim, B.H.; Kim, I.H. Concentrations of tocols and gamma-oryzanol compounds in rice bran oil obtained by fractional extraction with supercritical carbon dioxide. J. Oleo Sci. 2014, 63, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Monroy, Y.M.; Rodrigues, R.A.F.; Sartoratto, A.; Cabral, F.A. Extraction of bioactive compounds from cob and pericarp of purple corn (Zea mays L.) by sequential extraction in fixed bed extractor using supercritical CO2, ethanol, and water as solvents. J. Supercrit. Fluids 2016, 107, 250–259. [Google Scholar] [CrossRef]
- Zeković, Z.; Pavlić, B.; Cvetanović, A.; Đurović, S. Supercritical fluid extraction of coriander seeds: Process optimization, chemical profile and antioxidant activity of lipid extracts. Ind. Crops Prod. 2016, 94, 353–362. [Google Scholar] [CrossRef]
- Aladić, K.; Jarni, K.; Barbir, T.; Vidović, S.; Vladić, J.; Bilić, M.; Jokić, S. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind. Crops Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. The combined extraction of polyphenols from grape marc: Ultrasound assisted extraction followed by supercritical CO2 extraction of ultrasound-raffinate. LWT Food Sci. Technol. 2015, 61, 98–104. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Microwave pretreatment of moringa oleifera seed: Effect on oil obtained by pilot-scale supercritical carbon dioxide extraction and soxhlet apparatus. J. Supercrit. Fluids 2016, 107, 38–43. [Google Scholar] [CrossRef]
- Lenucci, M.S.; De Caroli, M.; Marrese, P.P.; Iurlaro, A.; Rescio, L.; Böhm, V.; Dalessandro, G.; Piro, G. Enzyme-aided extraction of lycopene from high-pigment tomato cultivars by supercritical carbon dioxide. Food Chem. 2015, 170, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Mishima, K.; Sharmin, T.; Ito, S.; Kawakami, R.; Kato, T.; Misumi, M.; Suetsugu, T.; Orii, H.; Kawano, H.; et al. Ultrasonically enhanced extraction of luteolin and apigenin from the leaves of Perilla frutescens (L.) britt. Using liquid carbon dioxide and ethanol. Ultrason. Sonochem. 2016, 29, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Chhouk, K.; Quitain, A.T.; Gaspillo, P.A.D.; Maridable, J.B.; Sasaki, M.; Shimoyama, Y.; Goto, M. Supercritical carbon dioxide-mediated hydrothermal extraction of bioactive compounds from garcinia mangostana pericarp. J. Supercrit. Fluids 2016, 110, 167–175. [Google Scholar] [CrossRef]
- Duba, K.S.; Fiori, L. Supercritical CO2 extraction of grape seed oil: Effect of process parameters on the extraction kinetics. J. Supercrit. Fluids 2015, 98, 33–43. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Sales, L.P.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Supercritical carbon dioxide extraction of capsicum peppers: Global yield and capsaicinoid content. J. Supercrit. Fluids 2013, 81, 210–216. [Google Scholar] [CrossRef]
- Mouahid, A.; Crampon, C.; Toudji, S.-A.A.; Badens, E. Effects of high water content and drying pre-treatment on supercritical CO2 extraction from dunaliella salina microalgae: Experiments and modelling. J. Supercrit. Fluids 2016, 116, 271–280. [Google Scholar] [CrossRef]
- Park, H.S.; Choi, H.-K.; Lee, S.J.; Park, K.W.; Choi, S.-G.; Kim, K.H. Effect of mass transfer on the removal of caffeine from green tea by supercritical carbon dioxide. J. Supercrit. Fluids 2007, 42, 205–211. [Google Scholar] [CrossRef]
- Özkal, S.G.; Yener, M.E. Supercritical carbon dioxide extraction of flaxseed oil: Effect of extraction parameters and mass transfer modeling. J. Supercrit. Fluids 2016, 112, 76–80. [Google Scholar] [CrossRef]
- Nejad-Sadeghi, M.; Taji, S.; Goodarznia, I. Optimization of supercritical carbon dioxide extraction of essential oil from dracocephalum kotschyi boiss: An endangered medicinal plant in iran. J. Chromat. A 2015, 1422, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Barroso, P.T.W.; de Carvalho, P.P.; Rocha, T.B.; Pessoa, F.L.P.; Azevedo, D.A.; Mendes, M.F. Evaluation of the composition of carica papaya l. Seed oil extracted with supercritical CO2. Biotechnol. Rep. 2016, 11, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.; Sultana, B.; Anwar, F.; Adnan, A.; Rizvi, S.S.H. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids 2015, 104, 122–131. [Google Scholar] [CrossRef]
- Dutta, S.; Bhattacharjee, P. Enzyme-assisted supercritical carbon dioxide extraction of black pepper oleoresin for enhanced yield of piperine-rich extract. J. Biosci. Bioeng. 2015, 120, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in berberis asiatica fruits using response surface methodology (rsm). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Belayneh, H.D.; Wehling, R.L.; Cahoon, E.; Ciftci, O.N. Extraction of omega-3-rich oil from camelina sativa seed using supercritical carbon dioxide. J. Supercrit. Fluids 2015, 104, 153–159. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wang, P.; Xiao, Y.; Liu, Q. Optimization of supercritical carbon dioxide extraction, physicochemical and cytotoxicity properties of gynostemma pentaphyllum seed oil: A potential source of conjugated linolenic acids. Sep. Purif. Technol. 2016, 159, 147–156. [Google Scholar] [CrossRef]
- Paula, J.T.; Paviani, L.C.; Foglio, M.A.; Sousa, I.M.O.; Cabral, F.A. Extraction of anthocyanins from arrabidaea chica in fixed bed using CO2 and CO2/ethanol/water mixtures as solvents. J. Supercrit. Fluids 2013, 81, 33–41. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Ganjloo, A.; Taip, F.S.; Salleh, L.M.; Sarker, M.Z.I. Optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from spearmint (mentha spicata l.) leaves by using response surface methodology. Food Bioprocess Technol. 2012, 5, 912–920. [Google Scholar] [CrossRef]
- Shao, Q.; Huang, Y.; Zhou, A.; Guo, H.; Zhang, A.; Wang, Y. Application of response surface methodology to optimise supercritical carbon dioxide extraction of volatile compounds from crocus sativus. J. Sci. Food Agric. 2014, 94, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- De Cássia Rodrigues Batista, C.; de Oliveira, M.S.; Araújo, M.E.; Rodrigues, A.M.C.; Botelho, J.R.S.; da Silva Souza Filho, A.P.; Machado, N.T.; Carvalho Junior, R.N. Supercritical CO2 extraction of açaí (Euterpe oleracea) berry oil: Global yield, fatty acids, allelopathic activities, and determination of phenolic and anthocyanins total compounds in the residual pulp. J. Supercrit. Fluids 2016, 107, 364–369. [Google Scholar] [CrossRef]
- Ghosh, S.; Chatterjee, D.; Das, S.; Bhattacharjee, P. Supercritical carbon dioxide extraction of eugenol-rich fraction from ocimum sanctum linn and a comparative evaluation with other extraction techniques: Process optimization and phytochemical characterization. Ind. Crops Prod. 2013, 47, 78–85. [Google Scholar] [CrossRef]
- Corzzini, S.C.S.; Barros, H.D.F.Q.; Grimaldi, R.; Cabral, F.A. Extraction of edible avocado oil using supercritical CO2 and a CO2/ethanol mixture as solvents. J. Food Eng. 2017, 194, 40–45. [Google Scholar] [CrossRef]
- Garmus, T.T.; Paviani, L.C.; Queiroga, C.L.; Cabral, F.A. Extraction of phenolic compounds from pepper-rosmarin (Lippia sidoides Cham.) leaves by sequential extraction in fixed bed extractor using supercritical CO2, ethanol and water as solvents. J. Supercrit. Fluids 2015, 99, 68–75. [Google Scholar] [CrossRef]
- Nunes da Ponte, M. Chapter 1 supercritical fluids in natural product and biomass processing—An introduction. In High Pressure Technologies in Biomass Conversion; The Royal Society of Chemistry: Cambridge, UK, 2017; pp. 1–8. [Google Scholar]
- Nobre, B.P.; Gouveia, L.; Matos, P.G.S.; Cristino, A.F.; Palavra, A.F.; Mendes, R.L. Supercritical extraction of lycopene from tomato industrial wastes with ethane. Molecules 2012, 17, 8397. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Lopes, A.M.; Brenner, M.; Falé, P.; Roseiro, L.B.; Bogel-Łukasik, R. Extraction and purification of phenolic compounds from lignocellulosic biomass assisted by ionic liquid, polymeric resins, and supercritical CO2. ACS Sustain. Chem. Eng. 2016, 4, 3357–3367. [Google Scholar] [CrossRef]
- Lu, Z.-M.; Xu, W.; Yu, N.-H.; Zhou, T.; Li, G.-Q.; Shi, J.-S.; Xu, Z.-H. Recovery of aroma compounds from zhenjiang aromatic vinegar by supercritical fluid extraction. Int. J. Food Sci. Technol. 2011, 46, 1508–1514. [Google Scholar] [CrossRef]
- Shen, Z.; Mishra, V.; Imison, B.; Palmer, M.; Fairclough, R. Use of adsorbent and supercritical carbon dioxide to concentrate flavor compounds from orange oil. J. Agric. Food Chem. 2002, 50, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Viganó, J.; Coutinho, J.P.; Souza, D.S.; Baroni, N.A.F.; Godoy, H.T.; Macedo, J.A.; Martínez, J. Exploring the selectivity of supercritical CO2 to obtain nonpolar fractions of passion fruit bagasse extracts. J. Supercrit. Fluids 2016, 110, 1–10. [Google Scholar] [CrossRef]
- Gracia, I.; Rodríguez, J.F.; de Lucas, A.; Fernandez-Ronco, M.P.; García, M.T. Optimization of supercritical CO2 process for the concentration of tocopherol, carotenoids and chlorophylls from residual olive husk. J. Supercrit. Fluids 2011, 59, 72–77. [Google Scholar] [CrossRef]
- Yee, J.L.; Khalil, H.; Jiménez-Flores, R. Flavor partition and fat reduction in cheese by supercritical fluid extraction: Processing variables. Lait 2007, 87, 269–285. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Adzahan, N.M.; Islam Sarker, M.Z.; Ganjloo, A. Supercritical carbon dioxide extraction of seed oil from winter melon (Benincasa hispida) and its antioxidant activity and fatty acid composition. Molecules 2013, 18, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Dal Prá, V.; Dolwitsch, C.B.; Da Silveira, G.D.; Porte, L.; Frizzo, C.; Tres, M.V.; Mossi, V.; Mazutti, M.A.; Do Nascimento, P.C.; Bohrer, D.; et al. Supercritical CO2 extraction, chemical characterisation and antioxidant potential of brassica oleracea var capitata against HO•, O2•−, and ROO•. Food Chem. 2013, 141, 3954–3959. [Google Scholar] [CrossRef] [PubMed]
- Przygoda, K.; Wejnerowska, G. Extraction of tocopherol-enriched oils from quinoa seeds by supercritical fluid extraction. Ind. Crops Prod. 2015, 63, 41–47. [Google Scholar] [CrossRef]
- Hurtado-Benavides, A.; Dorado, D.A.; Sánchez-Camargo, A.D.P. Study of the fatty acid profile and the aroma composition of oil obtained from roasted colombian coffee beans by supercritical fluid extraction. J. Supercrit. Fluids 2016, 113, 44–52. [Google Scholar] [CrossRef]
- Barbosa, H.M.A.; De Melo, M.M.R.; Coimbra, M.A.; Passos, C.P.; Silva, C.M. Optimization of the supercritical fluid coextraction of oil and diterpenes from spent coffee grounds using experimental design and response surface methodology. J. Supercrit. Fluids 2014, 85, 165–172. [Google Scholar] [CrossRef]
- Singh, A.; Ahmad, A.; Bushra, R. Supercritical carbon dioxide extraction of essential oils from leaves of eucalyptus globulus l., their analysis and application. Anal. Methods 2016, 8, 1339–1350. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; De Melo, M.M.R.; Oliveira, E.L.G.; Neto, C.P.; Silvestre, A.J.D.; Silva, C.M. Optimization of the supercritical fluid extraction of triterpenic acids from eucalyptus globulus bark using experimental design. J. Supercrit. Fluids 2013, 74, 105–114. [Google Scholar] [CrossRef]
- Garmus, T.T.; Paviani, L.C.; Queiroga, C.L.; Magalhães, P.M.; Cabral, F.A. Extraction of phenolic compounds from pitanga (Eugenia uniflora L.) leaves by sequential extraction in fixed bed extractor using supercritical CO2, ethanol and water as solvents. J. Supercrit. Fluids 2014, 86, 4–14. [Google Scholar] [CrossRef]
- Larkeche, O.; Zermane, A.; Meniai, A.H.; Crampon, C.; Badens, E. Supercritical extraction of essential oil from juniperus communis l. Needles: Application of response surface methodology. J. Supercrit. Fluids 2015, 99, 8–14. [Google Scholar] [CrossRef]
- Filip, S.; Djarmati, Z.; Lisichkov, K.; Csanadi, J.; Jankov, R.M. Isolation and characterization of maclura (Maclura pomifera) extracts obtained by supercritical fluid extraction. Ind. Crops Prod. 2015, 76, 995–1000. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. A parametric study of supercritical carbon dioxide extraction of oil from moringa oleifera seeds using a response surface methodology. Sep. Purif. Technol. 2013, 113, 9–17. [Google Scholar] [CrossRef]
- Ruttarattanamongkol, K.; Siebenhandl-Ehn, S.; Schreiner, M.; Petrasch, A.M. Pilot-scale supercritical carbon dioxide extraction, physico-chemical properties and profile characterization of moringa oleifera seed oil in comparison with conventional extraction methods. Ind. Crops Prod. 2014, 58, 68–77. [Google Scholar] [CrossRef]
- Sajfrtova, M.; Sovova, H.; Karban, J. Enrichment of nigella damascena extract with volatile compounds using supercritical fluid extraction. J. Supercrit. Fluids 2014, 94, 160–164. [Google Scholar] [CrossRef]
- Ben Rahal, N.; Barba, F.J.; Barth, D.; Chevalot, I. Supercritical CO2 extraction of oil, fatty acids and flavonolignans from milk thistle seeds: Evaluation of their antioxidant and cytotoxic activities in Caco-2 cells. Food Chem. Toxicol. 2015, 83, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Filip, S.; Vidović, S.; Vladić, J.; Pavlić, B.; Adamović, D.; Zeković, Z. Chemical composition and antioxidant properties of ocimum basilicum l. Extracts obtained by supercritical carbon dioxide extraction: Drug exhausting method. J. Supercrit. Fluids 2016, 109, 20–25. [Google Scholar] [CrossRef]
- Marques, L.L.M.; Panizzon, G.P.; Aguiar, B.A.A.; Simionato, A.S.; Cardozo-Filho, L.; Andrade, G.; de Oliveira, A.G.; Guedes, T.A.; Mello, J.C.P.D. Guaraná (Paullinia cupana) seeds: Selective supercritical extraction of phenolic compounds. Food Chem. 2016, 212, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.A.; Bhusari, S.S.; Shinde, D.B.; Wakte, P.S. Optimization of process variables for phyllanthin extraction from phyllanthus amarus leaves by supercritical fluid using a box-behnken experimental design followed by hplc identification. Acta Pharm. 2013, 63, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Abdul Manap, M.Y.B.; Solati, Z. Antioxidant activity of piper nigrum l. Essential oil extracted by supercritical CO2 extraction and hydro-distillation. Talanta 2014, 121, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.A.; Cardoso, M.D.G.; Guimarães, L.G.L.; Queiroz, F.; Barbosa, L.C.A.; Morais, A.R.; Nelson, D.L.; Andrade, M.A.; Zacaroni, L.M.; Pimentel, S.M.N.P. Extracts from the leaves of piper piscatorum (trel. Yunc.) obtained by supercritical extraction of with CO2, employing ethanol and methanol as co-solvents. Ind. Crops Prod. 2013, 43, 490–495. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Srivastav, P.P.; Mishra, H.N. Optimization of process variables for supercritical fluid extraction of ergothioneine and polyphenols from pleurotus ostreatus and correlation to free-radical scavenging activity. J. Supercrit. Fluids 2014, 95, 51–59. [Google Scholar] [CrossRef]
- Cui, W.; Ruan, X.; Li, Z.; Zhang, H.; Liu, B.; Wang, Q. Optimization of process parameters of extraction of lotaustralin from the roots of rhodiola rosea l using supercritical CO2 plus modifier. Toxicol. Environ. Chem. 2016, 98, 727–735. [Google Scholar]
- Salgın, U.; Salgın, S.; Ekici, D.D.; Uludağ, G. Oil recovery in rosehip seeds from food plant waste products using supercritical CO2 extraction. J. Supercrit. Fluids 2016, 118, 194–202. [Google Scholar] [CrossRef]
- Zulkafli, Z.D.; Wang, H.; Miyashita, F.; Utsumi, N.; Tamura, K. Cosolvent-modified supercritical carbon dioxide extraction of phenolic compounds from bamboo leaves (Sasa palmata). J. Supercrit. Fluids 2014, 94, 123–129. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Water and ethanol as co-solvent in supercritical fluid extraction of proanthocyanidins from grape marc: A comparison and a proposal. J. Supercrit. Fluids 2014, 87, 1–8. [Google Scholar] [CrossRef]
- Patil, A.A.; Sachin, B.S.; Wakte, P.S.; Shinde, D.B. Optimization of supercritical fluid extraction and hplc identification of wedelolactone from wedelia calendulacea by orthogonal array design. J. Adv. Res. 2014, 5, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Mesomo, M.C.; Corazza, M.L.; Ndiaye, P.M.; Dalla Santa, O.R.; Cardozo, L.; Scheer, A.D.P. Supercritical CO2 extracts and essential oil of ginger (Zingiber officinale r.): Chemical composition and antibacterial activity. J. Supercrit. Fluids 2013, 80, 44–49. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Filho, W.A.R.; Paula, J.T.; Garmus, T.T.; Cabral, F.A. Solubility of γ-oryzanol in supercritical carbon dioxide and extraction from rice bran. J. Supercrit. Fluids 2016, 107, 196–200. [Google Scholar] [CrossRef]
- Zuknik, M.H.; Nik Norulaini, N.A.; Wan Nursyazreen Dalila, W.S.; Ali, N.R.; Omar, A.K.M. Solubility of virgin coconut oil in supercritical carbon dioxide. J. Food Eng. 2016, 168, 240–244. [Google Scholar] [CrossRef]
- Roy, B.C.; Goto, M.; Hirose, T. Extraction of ginger oil with supercritical carbon dioxide: Experiments and modeling. Ind. Eng. Chem. Res. 1996, 35, 607–612. [Google Scholar] [CrossRef]
- Lalas, S.; Tsaknis, J. Characterization of moringa oleifera seed oil variety “periyakulam 1”. J. Food Compos. Anal. 2002, 15, 65–77. [Google Scholar] [CrossRef]
- Nagy, B.; Simándi, B. Effects of particle size distribution, moisture content, and initial oil content on the supercritical fluid extraction of paprika. J. Supercrit. Fluids 2008, 46, 293–298. [Google Scholar] [CrossRef]
- Balasubramanian, R.K.; Yen Doan, T.T.; Obbard, J.P. Factors affecting cellular lipid extraction from marine microalgae. Chem. Eng. J. 2013, 215–216, 929–936. [Google Scholar] [CrossRef]
- Crampon, C.; Mouahid, A.; Toudji, S.-A.A.; Lépine, O.; Badens, E. Influence of pretreatment on supercritical CO2 extraction from nannochloropsis oculata. J. Supercrit. Fluids 2013, 79, 337–344. [Google Scholar] [CrossRef]
- Ivanovic, J.; Ristic, M.; Skala, D. Supercritical CO2 extraction of helichrysum italicum: Influence of CO2 density and moisture content of plant material. J. Supercrit. Fluids 2011, 57, 129–136. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. Effect of ultrasound pre-treatment of hemp (Cannabis sativa L.) seed on supercritical CO2 extraction of oil. J. Food Sci. Technol. 2015, 52, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Said, P.P.; Arya, O.P.; Pradhan, R.C.; Singh, R.S.; Rai, B.N. Separation of oleoresin from ginger rhizome powder using green processing technologies. J. Food Process Eng. 2015, 38, 107–114. [Google Scholar] [CrossRef]
- Chemat, F.; Zill e, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Riera, E.; Golas, Y.; Blanco, A.; Gallego, J.A.; Blasco, M.; Mulet, A. Mass transfer enhancement in supercritical fluids extraction by means of power ultrasound. Ultrason. Sonochem. 2004, 11, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Ding, C.; Hu, A.; Yan, J. Analyses of factors affecting the ultrasonically—Enhanced supercritical fluid extraction of epa and dha from algae. J. South China Univ. Technol. (Nat. Sci.) 2004, 32, 43–47. [Google Scholar]
- Wei, M.C.; Xiao, J.; Yang, Y.C. Extraction of α-humulene-enriched oil from clove using ultrasound-assisted supercritical carbon dioxide extraction and studies of its fictitious solubility. Food Chem. 2016, 210, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.B.; Arroio Sergio, C.S.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L. Var. Pendulum). Ultrason. Sonochem. 2016, 31, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Aguiar, A.C.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound. Ultrason. Sonochem. 2015, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.C.; Yang, Y.C. Kinetic studies for ultrasound-assisted supercritical carbon dioxide extraction of triterpenic acids from healthy tea ingredient hedyotis diffusa and hedyotis corymbosa. Sep. Purif. Technol. 2015, 142, 316–325. [Google Scholar] [CrossRef]
- Balachandran, S.; Kentish, S.E.; Mawson, R.; Ashokkumar, M. Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006, 13, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.M.; Dalmolin, I.; Carareto, N.D.D.; Basso, R.C.; Meirelles, A.J.A.; Vladimir Oliveira, J.; Batista, E.A.C.; Meireles, M.A.A. Supercritical fluid extraction of grape seed: Process scale-up, extract chemical composition and economic evaluation. J. Food Eng. 2012, 109, 249–257. [Google Scholar] [CrossRef]
- Zabot, G.L.; Moraes, M.N.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds from botanic matrices: Experimental data, process parameters and economic evaluation. Recent Pat. Eng. 2012, 6, 182–206. [Google Scholar] [CrossRef]
- Prado, J.M.; Prado, G.H.C.; Meireles, M.A.A. Scale-up study of supercritical fluid extraction process for clove and sugarcane residue. J. Supercrit. Fluids 2011, 56, 231–237. [Google Scholar] [CrossRef]
- Rocha-Uribe, J.A.; Novelo-Pérez, J.I.; Araceli Ruiz-Mercado, C. Cost estimation for CO2 supercritical extraction systems and manufacturing cost for habanero chili. J. Supercrit. Fluids 2014, 93, 38–41. [Google Scholar] [CrossRef]
- Paula, J.T.; Aguiar, A.C.; Sousa, I.M.O.; Magalhães, P.M.; Foglio, M.A.; Cabral, F.A. Scale-up study of supercritical fluid extraction process for baccharis dracunculifolia. J. Supercrit. Fluids 2016, 107, 219–225. [Google Scholar] [CrossRef]
- Pronyk, C.; Mazza, G. Design and scale-up of pressurized fluid extractors for food and bioproducts. J. Food Eng. 2009, 95, 215–226. [Google Scholar] [CrossRef]
- Schwartzberg, H.G.; Chao, R.Y. Solute diffusivities in leaching processes. Food Technol. 1982, 36, 73–76. [Google Scholar]
- Kubátová, A.; Jansen, B.; Vaudoisot, J.-F.; Hawthorne, S.B. Thermodynamic and kinetic models for the extraction of essential oil from savory and polycyclic aromatic hydrocarbons from soil with hot (subcritical) water and supercritical CO2. J. Chromatogr. A 2002, 975, 175–188. [Google Scholar] [CrossRef]
- Passos, C.P.; Coimbra, M.A.; Da Silva, F.A.; Silva, C.M. Modelling the supercritical fluid extraction of edible oils and analysis of the effect of enzymatic pre-treatments of seed upon model parameters. Chem. Eng. Res. Des. 2011, 89, 1118–1125. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Domingues, R.M.A.; Sova, M.; Lack, E.; Seidlitz, H.; Lang Jr, F.; Silvestre, A.J.D.; Silva, C.M. Scale-up studies of the supercritical fluid extraction of triterpenic acids from eucalyptus globulus bark. J. Supercrit. Fluids 2014, 95, 44–50. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Parjikolaei, B.R.; Lari, H.N.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Pilot-plant scale extraction of phenolic compounds from mango leaves using different green techniques: Kinetic and scale up study. Chem. Eng. J. 2016, 299, 420–430. [Google Scholar] [CrossRef]
- Prado, I.M.; Prado, G.H.C.; Prado, J.M.; Meireles, M.A.A. Supercritical CO2 and low-pressure solvent extraction of mango (Mangifera indica) leaves: Global yield, extraction kinetics, chemical composition and cost of manufacturing. Food Bioprod. Process. 2013, 91, 656–664. [Google Scholar] [CrossRef]
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Knez Hrnčič, M.; Škerget, M. Industrial applications of supercritical fluids: A review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Knez, Ž.; Škerget, M.; Hrňič, M. Principles of supercritical fluid extraction and applications in the food, beverage and nutraceutical industries. Sep. Extr. Conc. Processes Food Beverage Nutr. Ind. 2010, 3–38. [Google Scholar] [CrossRef]
- Khosravi-Darani, K. Research activities on supercritical fluid science in food biotechnology. Crit. Rev. Food Sci. Nutr. 2010, 50, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Askin, R.; Ötles, S. Supercritical fluids. Acta Sci. Pol. Technol. Aliment. 2005, 4, 3–16. [Google Scholar]
- Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M.J.; Linhardt, R.J. Green solvents in carbohydrate chemistry: From raw materials to fine chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef] [PubMed]

| Method | Advantages | Disadvantages |
|---|---|---|
| Microwave-assisted extraction (used with traditional methods) | rapid extraction; small amount of solvent; relatively low additional costs | use of high pressure and temperature; limited amount of sample; non-selective (large number of compounds extracted) |
| Supercritical fluid extraction (SFE) methods | rapid extraction; small amount of organic solvent or no solvent; no solvent residue; preserves thermally labile compounds; tunable solvent (SCF) density; selective extraction (small number of compounds extracted); inexpensive to operate/run | high setup cost; technical knowledge of SCF properties required (e.g., phase behaviour, cross-over region) |
| Mechanical extraction | mainly for extraction of oil and juice; does not require external heat and solvent | limited application and non-selective |
| Ultrasound-assisted extraction (used with traditional methods) | rapid extraction; small amount of solvent; relatively low additional cost | non-selective |
| DIC extraction—détente instantanée controlee (steam driven with rapid depressurisation) | improved extraction yield; rapid extraction | high cost; high energy consumption; high temperature; preferably used for sample pre-treatment process |
| Phase | Physical Property | ||
|---|---|---|---|
| Density × 102 (kg·m−3) | Diffusivity × 10−3 (cm2·s−1) | Viscosity × 10−4 (kg·m·s−1) | |
| Liquid | 6–16 | <0.005 | 2–30 |
| SCF | |||
| Pc, Tc | 2–5 | 0.7 | 0.1–0.3 |
| 4Pc, Tc | 4–9 | 0.2 | 0.3–0.9 |
| Gas | 0.006–0.02 | 0.1–0.4 | 0.1–0.3 |
| SCF | Molecular Weight | Critical Temperature | Critical Pressure | Density at CP † | Notes |
|---|---|---|---|---|---|
| g·mol−1 | °C | bar (psi) * | kg·m−3 | ||
| Air | n/a | −140.6 | 37.7 (546.8) | 319.9 | Green technology fluids and relatively higher CP densities |
| Ammonia (NH3) | 17.03 | 132.2 | 113.3 (1643.2) | 225 | |
| Nitrogen (N2) | 28.01 | −147 | 34 (493.1) | 313.3 | |
| Water (H2O) | 18.02 | 373.9 | 220.6 (3166) | 322 | |
| Carbon dioxide (CO2) | 44.01 | 30.9 | 73.7 (1056) | 467.6 | Greener ** technology and high CP density |
| Chlorotrifluoromethane (CCIF3) | 104.5 | 28.8 | 38.8 (563.3) | 582.9 | Higher CP densities but environmentally hazardous |
| Dichlorofluoromethane (CHCl2F) | 102.9 | 178.3 | 51.8 (751.3) | 526.1 | |
| Octafluoropropane (C3F8) | 188 | 71.9 | 26.8 (388.7) | 629 | |
| Acetone (C3H6O) | 58.08 | 235.1 | 46.4 (672.9) | 278 | Lower CP densities and environmentally hazardous |
| Benzene (C6H6) | 78.11 | 289 | 49 (710.7) | 30.9 | |
| Dimethyl Ether (CH3)2O | 46.1 | 127.1 | 53.4 (774.5) | 277 | |
| Ethane (C2H6) | 30.07 | 32.2 | 48.7 (697.6) | 206.2 | |
| Ethanol (C2H5OH) | 46.07 | 240.9 | 60.6 (878.9) | 276 | |
| Ethylene (C2H4) | 28.05 | 9.2 | 50.4 (720.9) | 214.2 | |
| Methane (CH4) | 16.04 | −82.6 | 45.9 (658.5) | 162.7 | |
| Methanol (CH3OH) | 32.04 | 239.4 | 81 (1157.4) | 275.5 | |
| n-Propane (C3H8) | 44.1 | 96.7 | 42.5 (761.4) | 220.5 | |
| Propylene (C3H6) | 42.08 | 91.9 | 45.5 (658.5) | 230.1 |
| Natural Source (Scientific Name) | SFE Conditions | Major Findings about the Extraction/Extract | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Pressure (bar) | Flowrate (g/min) | Processing Time (min) | Dimension Extractor (D or ID × L) | Solvent/Solid Ratio (gCO2/g) * | Identified Molecule/s | Amount of Active | Reference | Remark/s | |
| Industrial examples | 35–38 | 248 | - | - | 7003 L | - | caffeine | - | [63] | Decaffeination of tea leaves by SFE, Evonik Industries AG (Essen Germany) |
| Wide variety of (Tea leaves) Theaceae | ||||||||||
| Industrial waste: Tomato skins and seeds | 60 | 300 | 0.16, 0.27, and 0.41 | 3–8 h | 5 mL ID = 7.9 mm | 220 | E-lycopene | 86% recovery of E-lycopene | [64] | Lab-bench scale and the result was from the CO2 only method |
| Agricultural by-product | 35 | 140 | 0.339 | - | 6 mL | - | lignin derived bioactive compounds e.g., tricin and catechins | - | [65] | SCF used as the separation of fractions from ionic liquids. Relatively poor extractions yields of flavonoids was explained by the higher polarity of catechins than vanillin-like compounds |
| Wheat Straw | ||||||||||
| Vinegar | 50 | 350 | 0.42 | 120 | - | - | 44 aroma compounds e.g., acetic acid, benzaldehyde, ethyl acetate | - | [66] | SFE is used to recover highly prized aromas from a Chinese vinegar |
| Zhenjiang | ||||||||||
| Orange peel | 35 | 131 | 33.33 | - | - | - | Wide range of oxygenated compounds | - | [67] | Used as a second step to recover flavor compounds from a silica gel bed |
| Citrus sinensis (Orange oil) | ||||||||||
| Passion fruit | 50–60 | 170–260 | 20.64 | - | 54.37 mL (30.3 mm × 75.4 mm) | tocopherols, unsaturated fatty acid and carotenoids | - | [68] | Sequential SFE method marked an increase in retrieval of bioactives compared to single step SFE method | |
| Passiflora edulis (Passion fruit bagasse) | ||||||||||
| Grape marc | 40 | 200–500 | 0.41–32.78 | - | Used a series of extractors (0.1, 0.2, and 0.5 L) | UFAs and vitamin E (tocopherols and tocotrienols) | - | [43] | Study investigated extraction kinetics using SFE and grape marc | |
| Grape seed oil | ||||||||||
| Olive husk | 40–60 | 205–350 | 15L/min | 180 | 0.94 L (40 mm × 75 mm) | tocopherols, carotenoids and chlorophylls | - | [69] | Two-to-four times increase in recovery of bioactive compounds compared to conventional method | |
| Oleaceae | ||||||||||
| Dairy | 35 and 40 | 200 and 350 | - | - | - | - | non-polar lipids; triacylglycerides and FFAs | - | [70] | Reduction of fat content by 51% for cheddar and 55% for Parmesan |
| Cheddar and Parmesan cheeses | ||||||||||
| Plant example | 40–50 | 300–400 | 0.39 | - | - | - | luteolin, carajurin, 3-desoxyanthocyanidin | 19 mg/g leaf | [56] | Authors explored selectivity of SFE in extraction of phenolic compounds |
| Arrabidaea chica | ||||||||||
| Benincasa hispida (Winter melon) | 46 | 244 | 10 | 97 | 500 mL | - | linoleic acid | 176 mg extract/g dried sample | [71] | Antioxidant activity of SFE extract was higher than in extracts derived from conventional methods |
| Brassica oleracea | 60 | 250 | 2 | 180 | 100 mL | 765.9 | sulforaphane and iberin nitrile | - | [72] | Dal Prá et al. investigated optimal conditions for extraction of antioxidant constituents of industrial interests |
| Capcicum frutescens | 40 | 250 | 11.4 | 320 | - | 268.2 | capsaicin | 32.8 mg/g | [44] | Freeze-drying is the optimum sample pretreatment method to recover of capsaicinoids |
| Cannabis sativa | 40–60 | 300–400 | 1.94 kg/h | - | - | - | tocopherol | 125.37 μg/g | [37] | Extraction of α-tocopherol and γ-tocopherol |
| Carica papaya | 80 | 200 | 16.45 mL/min | 180 | 42 mL | 1180.4 | benzyl isothiocyanate | - | [49] | SFE is used to recover highly active compound from papaya seeds |
| Camelina sativa | 70 | 450 | 1 L/min | 510 | - | 16.14 | α-linoleic, oleic, eicosaenoic and erusic acids | - | [54] | SFE method is more efficient in recovery of oil compared to hexane extraction and cold press |
| Chenopodium Quinoa | 130 | 185 | 0.175–0.45 | 55–180 | 1.2 mL (0.5 cm × 6.1 cm) | 8.02 to 67.5 | tocopherol | 201.3 mg/100 g | [73] | Four times increase in vitamin E yield compared with hexane extraction |
| Chelidonium majus | 55 | 120 | 4.8 | - | 20 mL | - | chelidonine, cheleritrine, sanquinarine and berberine | - | [31] | Highly selective in extraction of chelidonine at solvent density of 813–850 kg/m3 |
| Coffea arabica | 35.9 | 331 | 70 | - | 1 L | - | palmitic, linoleic, oleic, strearic and arachidic acid, furans and pyrazine | - | [74] | Volatile compounds of furan and pyrazine type recovered from coffee beans |
| Crocus sativus | 44.9 | 349 | 10.1 L/h | 150 | - | 1377.27 | - | - | [58] | Pressure and flowrate had significant effects on recovery of volatile compounds |
| Dracocephalum kotschyi | 60 | 240 | 600 | 100 | 8.8 mm × 560 mm | 22,058 | citral, p-mentha-1,3,8-triene, D-3-carene and methyl geranate | - | [48] | CO2 and flowrate influence the extraction yield |
| Expresso Spent coffee ground | 55 | 190 | 12 | - | - | - | diterpenes | 102.9 mg/g | [75] | Yield of diterpenes increased by 212–410% |
| Eucalyptus globule | 80 | 350 | 12 | 60–120 | 200 mL | 60.15 to 400 | † DPPH (antioxidant) and superoxide anion scavenging | 26.05 and 47.61 μg/mL | [76] | Increase in temperature and flowrate augments the solubility and interaction of CO2 and essential oil |
| Eucalyptus globulus | 40 | 200 | 6 | 360 | - | 1800 | germacrenos D, germ-acrenos B + bicycle-germacrene, selina-1,3,7(11) -trien-8-one, selina-1,3,7(11)-trien-8-one epoxide, trans-caryophyllene | - | [77] | Study reported the recovery of triterpenic acids by 79.2% compared to soxhlet extraction |
| Eugenia uniflora | 60 | 400 | 2.4 | 360 | - | 20.09 | o-cymene, 1,8-cineole γ-terpinene, cis-sabinene (trans-4-thujanol), thymol-methyl ether, thymol carvacrol, α-copaene-trans-caryophyllene, germacrene D bicycle-germacrene, δ-cadinene, monoterpenes, sesquiterpenes | - | [78] | The authors showed that sequential extraction method is more effective in obtaining compounds of interest |
| Euterpe oleracea (Residual pulp) | FF | 490 | ~5.4 | 180 | - | 21.41 | linolenic, linoleic, oleic, and palmitic acids | - | [59] | The extracts obtained were more concentrated in monounsaturated fatty acid than polyunsaturated fatty acid |
| Garcinia mangostana | 140–160 | 50–100 | 2 mL/min | 30–60 | 8.8 mL | 1.5–3 | α-mangostin | - | [42] | SFE is used as means to extract α-mangostin with a yield of 0.203% |
| Gynostemma pentaphyllum | 43 | 320 | 333.33 | 160 | - | 1483.11 | linolenic acid | - | [55] | High content of unsaturated fatty acids is discovered (95.69%) compared with conventional methods |
| Juniperus communis | 55 | 300 | 7 | 60 | - | 64.12 | germacrene D and octadecene | - | [79] | Yield of seed oil is increased with sample particle diameter <0.315 mm |
| Lippia sidoides (sequential extraction) | 60 | 400 | 0.5 | 360 | - | 4.19 | - | - | [62] | Sequential extraction employing supercritical CO2 is effective in extraction of compounds of interest |
| Maclura pomifera | 40 | 210 | 333.33 | 360 | 4 L | 132.98 | lupeol ester of 3-hydroxyhexadecanoic acid | - | [80] | Extraction of new compound, 3-hydroxyhexadecanoic acid |
| Moringa oleifera | 60 | 500 | 2 mL/min | 120 | 50 mL | 37.85 | 1,2-benzenedicarboxylic acid, mono-(2-ethylhexyl) ester, nonacosane, heptacosane and β-amyrin | - | [81] | This work proved selectivity of SFE process by extracting 12 compounds compared to 42 compounds extracted by soxhlet extraction |
| Moringa oleifera | 30 | 350 | 333.33 | 300 | 2 L | 1329.77 | oleic acid, tocopherols and sterol | - | [82] | A health promoting fatty acid; Oleic acid (72.26–74.72%) is extracted by SFE process |
| Moringa oleifera (microwave pretreatment) | 40 | 300 | 166.7 | 210 | 1 L | 921.23–1000.2 | myristic acid, palmitic palmitoleic acid, stearic acid, oleic acid, linoleic acid, linolenic acid, arachidic acid, eicosenoic acid, behenic acid, lignoceric acid, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids | - | [39] | Microwave irradiation technique is used as the sample pretreatment step for SFE and soxhlet extraction, and SFE extract is claimed to be of higher quality than the soxhlet extract |
| Nigella damascena | 40 | 300 | 0.8 | 15 | 150 mL (ID = 30 mm) | 0.8 | germacrene A, damascenine and β-elemene | - | [83] | The yields of germacrene A and damascenine from SFE were 20% higher than with soxhlet extraction |
| Silybum marianum (milk thistle) | 40 | 220 | 5 mL/min | 150 | 150 mL (ID = 30 mm) | 23.56 | linoleic, oleic, palmitic acids, silychristin, silydianin, silibinin and taxifolin | - | [84] | The SFE extract showed potent cytotoxic effect against CaCo-2 cells |
| Ocimum basilicum | 60 | 150 | 3.22 | 240 | 200 mL | 91.89 | linalool, eugenol, α-begamotene, germacrene D, γ-cadinene, δ-cardinene, β-selinene | - | [85] | This study used a drug exhaustion method to extract non-polar compounds |
| Ocimum sanctum | 70 | 400 | - | 90 | - | - | eugenol | 0.463 g/100 g dry powder | [60] | SFE is used to obtain eugenol rich fraction as dry powder |
| Paullinia cupana | 40 | 100 | 40 | 25 mL | - | - | 20 mg/100 g | [86] | Ethanol is used as co-solvent to improve total phenolic content | |
| Persea Americana (Avocado) | 60 | 400 | - | - | - | α-tocopherol | - | [61] | 98% recovery of avocado oil from SFE extract was reported | |
| Phyllanthus amarus | 40 | 232 | 90 | 15 mm × 150 mm | - | phyllanthin | 12.83 mg/g | [87] | Co-solvent concentration and extraction time significantly effect extraction yield | |
| Piper nigrum | 50 | 300 | 2 mL/min | 80 | 50 mL | 74.07 | β-caryophyllene, limonene, cabinene, 3-carene and α-pinene | - | [88] | SFE extract exhibited a stronger radical scavenging activity compared with extract from hydrodistillation with EC50 of 103.28 and 316.27 µg /mL, respectively. Optimum parameter for antioxidant activity: T: 40 °C, time: 60 min |
| Piper piscatorum | 40 | 400 | 3.6 | 30 | - | 35.18 | piperovatine, palmitic acid, pentadecane, pipercallosidine | - | [89] | Chemical composition of extracts, particularly those containing amides was reduced when samples were air-dried |
| Pleurotus ostreatus (Mushroom) | 48 | 210 | 333.33 | 90 | 100 mL | 222,220 | phenol content: 5.48 mg GAE/g (dry weight) | 0.135 g dry weight | [90] | This study showed a good correlation between ergothioneine and † DPPH scavenging activity |
| Rhodiola rosea | 62 | 317 | 0.4 mL/min | 90 | 10 mL 60 mm × 15 mm | - | lotaustralin | 2.05 g/kg | [91] | SFE is used to extract cynogenic glucoside compound “lotaustralin” |
| Rosa canina (waste product) | 40 | 355 | 0.75 mL/min | 90 | 10 mL | 4090.91 | palmitic acid, stearic acid, oleic acid, linoleic acid, arachidonic acid | 0.0165 g dry solid | [92] | The highest extraction yield of oil was 16.5 g oil/100 g of dry solid |
| Sasa palmate | 95 | 200 | 10 mL/min | 9.65 mm × 45 mm | - | DL-alanine, gluconic acid, phosphoric acid, β-sitosterol, β-amyrene, α-amyrin acetate and friedelin | 0.73 g catechin equivalent | [93] | The used of mixture of co-solvent in 1:3 ratio enhanced yield of polyphenols and radical scavenging activity | |
| Vitis vinefera (grape marc) | 40–60 | 251 | 167.7 | 180 | - | 2795 | - | 10.8 g/100 g | [94] | 15% water as co-solvent efficiently extracts polar polyphenols from grape marc |
| Wedelia calendulacea | 40 | 250 | 90 | - | - | wedelolactone | 0.008 g/100 g | [95] | SFE method is more selective to extract wedelolactone compared with Soxhlet extraction | |
| Zingiber officinale | 50 | 250 | 2 cm3/min | 180 | 150 mL 25.2 mm × 290 mm | 137.4 | α-zingiberene, β-sesquiphellandrene, α-farnesene, geranial, β-bisabolene and β-eudesmol | 2.62 g/100 g | [96] | SFE extract showed higher capacity in antimicrobial activity than hydrodistillation |
| Sample | SFE Conditions | Device/Sonication Power | Remark | Ref. | |||
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Pressure (bar) | Flow Rate (kg/s) | Time (min) | ||||
| Syzygium aromaticum | 32 | 95 | 0.233 × 10−4 | 115 | ultrasonic bath at 185 W | Yield of clove oil 11% higher by ultrasonic assisted SPE and 1.2 times increase in extraction of α-humulene | [110] |
| Capsicum baccatum | 40 | 250 | 1.75 × 10−4 | 80 | ultrasound probe at 600 W | Yield of capsai- cinoid increased up to 12%. Global yield increased up to 45% | [111] |
| Capsicum frutescens | 40 | 150 | 1.673 × 10−4 | 60 | ultrasound probe at 360 W | Global yield increased up to 77% | [112] |
| Hedyotis diffusa | 55 | 245 | - | 95 | ultrasonic bath at 185 W | Yield increased by 11–14% | [113] |
| Perilla frutescens | 25 | 100 | - | 60 | ultrasound irradiation 125 s | Yield of luteolin increased by 53% Yield of apigenin increased by 144% | [41] |
| Almond Oil (source unknown) | 55 | 280 | 55.6 × 10−4 | 510 | ultrasonic probe | Extraction yield of the oil was enhanced by 20% | [108] |
| Zingiber Officinale | 40 | 160 | - | 200 | unknown device at 300 W | Yield: 30% higher | [114] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. https://doi.org/10.3390/molecules22071186
Khaw K-Y, Parat M-O, Shaw PN, Falconer JR. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules. 2017; 22(7):1186. https://doi.org/10.3390/molecules22071186
Chicago/Turabian StyleKhaw, Kooi-Yeong, Marie-Odile Parat, Paul Nicholas Shaw, and James Robert Falconer. 2017. "Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review" Molecules 22, no. 7: 1186. https://doi.org/10.3390/molecules22071186
APA StyleKhaw, K. -Y., Parat, M. -O., Shaw, P. N., & Falconer, J. R. (2017). Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules, 22(7), 1186. https://doi.org/10.3390/molecules22071186