1. Introduction
Uterine contractions with low frequency and amplitude occurs throughout the entire menstrual cycle. However, the amplitude increases dramatically at the time of menstruation for patients with primary dysmenorrhea (PD), which subsequently has a significant negative impact on their study, work and normal lives [
1]. Moreover, severe uterine contractions during premature labor even results in a high risk of newborn deaths [
2,
3]. Consequently, the relaxation of uterine spasm is a crucial strategy for the treatment of PD and premature labor.
Heat shock protein 27 (HSP27), a member of the small heat shock proteins family with a low-molecular weight, is a 15–30 kDa protein encoded by the
HSPB1 gene and typically exists as large, polydisperse assemblies [
4,
5]. HSP27 appears in all types of muscle cells with multiple functions of ATP-independent chaperone activity, thermo-tolerance, regulation of cell differentiation, cell migration, apoptosis, membrane stability, actin polymerization and muscle contraction [
6]. Recently, it has been put forward that the phosphorylation of HSP27 is associated with the change of quaternary structure and plays an important role in regulation of uterine contractions. It has been regarded as a potential target for the relevant diseases such as labor and PD [
3].
Licorice is derived from the roots and rhizomes of
Glycyrrhiza uralensis Fisch. (Fabaceae), a leguminous perennial herb. It is a commonly used Chinese herbal medicine with the main indications of detoxification, relieving cough and reducing sputum, relieving sore-throat, hepatoprotection, analgesia, etc. [
7,
8,
9,
10]. The extracts used from
Glycyrrhiza uralensis have a complex chemical composition and more than 400 compounds have been identified from Glycyrrhiza species, including flavonoids, triterpenoid saponins, coumarin and chalcones [
11]. The major compounds, such as glycyrrhizin, liquiritin, liquiritigenin and isoliquiritigenin, have been reported to exert a variety of biological activities including being anti-inflammatory, antidiabetic, antibacterial, antioxidant, anticancer and antispasmodic [
12,
13]. Jia et al. demonstrated that an aqueous licorice extract exerted spasmolytic effects on isolated mouse uteri, of which contractions were aroused by various stimulants, including potassium chloride, acetylcholine, carbachol, oxytocin or bradykinin. However, little is known about its molecular mechanism and bioactive constituents [
14,
15].
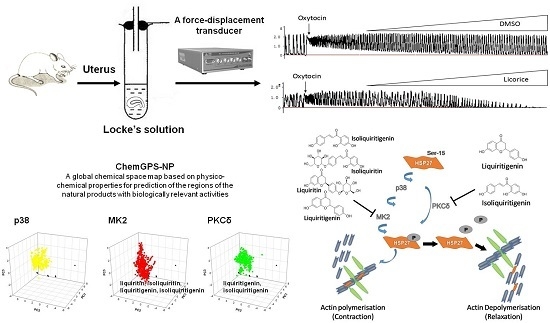
In the present study, the spasmolytic efficacy of aqueous licorice extract was monitored by a force-displacement transducer on an ex vivo model of oxytocin-induced uterine contraction. Simultaneously, the morphological change of myometrial smooth muscle cells was performed by histological examination. The phosphorylation of heat shock protein 27 (HSP27) was studied to explore the underlying molecular mechanism of licorice on spasmolysis. Moreover, UHPLC-Q Extractive Orbitrap-HRMS analysis was used to identify the seven principal chemical constituents in the licorice aqueous extract. ChemGPS-NP, a tool for navigating the chemical property space of natural products, was used to predict the biological activities of the constituents in the licorice aqueous solution [
16,
17]. Subsequently, the potential of the predicted bioactive ingredients as relevant up-stream kinase inhibitors was scrutinized by molecular docking.
3. Discussion
Uterine hypercontractility induces the painful menstrual cramps of adolescents and even results in a high risk of newborn deaths during premature labor. Licorice is one of the most commonly-used Chinese herbal medicine and has a long history of treating spastic pain. The present study showed that the aqueous extract from licorice significantly inhibited the amplitude and frequency of oxytocin-stimulated uterine contractions in a concentration-dependent manner. From the morphological examination, licorice obviously maintained the fusiform-shaped morphology of myometrial smooth muscle cells with regular arrangement and decreased the proportion of contractile cells in uterine inner annular layers, which was consistent with the functional evaluation.
HSP27 is a uterine contraction-related protein. These proteins can directly interact with each other to form homo-oligomers or hetero-oligomeric complexes to modulate actin filament formation, dynamics and contraction. These quaternary structures are tightly associated with its function and can be regulated by phosphorylation [
18,
19]. Actually, the phosphorylation of HSP27 can result in the destabilization and dissociation of the HSP27 oligomer, shifting the equilibrium into small monomer or dimer subunits. Furthermore, this can induce the alteration of the tertiary structure to change its interaction with the actin cytoskeleton [
20,
21,
22]. Eventually, the phosphorylation of HSP27 on serine residue 15 increases its stability and propensity to bind to actin filaments, which can promote uterine contraction and is involved in various human diseases such as labor and primary dysmenorrhea [
2,
3]. In the present study, the percentage of phosphorylation of HSP27 at Ser-15 residue increased up to 50.33% at 60 min after oxytocin stimulation and the increase was significantly suppressed by licorice. Colocalization between HSP27 and α-SMA was observed in the myometrial tissues, especially along the actin bundles in the oxytocin-stimulated model group. On the contrary, it was no longer colocalized after treatment with licorice. These results demonstrated that licorice can exert spasmolytic effects through inhibiting the phosphorylation of HSP27 to alter the interaction between HSP27 and actin in addition to probably promoting the non-phosphorylated HSP27 to sequester actin monomers, thus decreasing actin polymerization.
In vivo, it has been proven that HSP27 can be phosphorylated at the serine residue 15 by protein kinase C (PKC), p38 mitogen-activated protein kinases (p38 MAPK) and MAP kinase-activated protein kinase 2 (MK2) [
23]. The signal transduction cascades involving p38 MAPK and MK2-regulated phosphorylation of HSP27 are important in modulating filament dynamics [
21]. PKCδ, the most efficient kinase in phosphorylating HSP27, indirectly phosphorylates HSP27 through activation of the p38 MAPK/MK2 pathway, which has been confirmed by Takai et al. [
24]. ChemGPS-NP is a global chemical space map based on physico-chemical properties, which is used for predicting the analogous and biologically relevant activities of the natural products [
16,
17]. This map can be economically and efficiently used for investigating the bioactive constituents from Chinese medicine characterized by multiple components, targets and pathways [
25]. In the present study, ChemGPS-NP was used to predict the specific bioactive constituents targeting the above-mentioned kinases responsible for the phosphorylation of HSP27 from the aqueous extract of licorice. As the results showed, liquiritigenin and isoliquiritigenin were preliminarily classified as PKCδ inhibitors while liquiritin, isoliquiritin, liquiritigenin and isoliquiritigenin as MK2 inhibitors depending on the similar physico-chemical properties referring to known kinase inhibitors. This predicted information explained the bioactive constituents of licorice extract responsible for the particular pharmacological mechanism regulating the phosphorylation of HSP27.
Moreover, the predicted bioactive ingredients were scrutinized by molecular docking as kinase inhibitors. The results showed each complex of the MK2 or PKC catalytic domain with the corresponding compound had a stable formation with low binding energy (
Figure 7 and
Figure 8). The interaction of hydrogen bonds at the specific active site residues (
Table 3 and
Table 4) indicated the high affinity and tight binding capacity as the relevant kinase inhibitors.
In conclusion, our results revealed that licorice exerted a uterine relaxant effect through inhibiting the phosphorylation of HSP27 to alter the interaction of HSP27 and actin, which was due to the possible inhibitory effect of particular bioactive constituents on the up-stream kinase of MK2 or PKC regulating the phosphorylation of HSP27 (
Figure 9).
4. Materials and Methods
4.1. Materials and Reagents
The licorice was purchased from Nanjing Traditional Chinese Medicine Out-patient Department (Nanjing, Jiangsu, China) and verified as un-compromised authentic crude drugs by the corresponding author Boyang Yu. Estradiol benzoate for injection was obtained from Tianjin Jinyao Amino Acid Pharmaceutical Co., Ltd. (Tianjin, China). Oxytocin was purchased from Nanjing Xinbai Pharmaceutical Co., Ltd. (Nanjing, Jiangsu, China). The HSP27, pHSP27-Ser15 and α-SMA primary antibodies, Alexa Fluor® 647 conjugated anti-rabbit and Alexa Fluor® 488 conjugated anti-mouse secondary antibodies were obtained from Abcam (Burlingame, CA, USA). Anti-fade mounting reagent, DAPI staining solution, radioimmunoprecipitation assay buffer (RIPA), protein assay kit (BCA), phosphate-buffered saline (PBS) and bovine serum albumin (BSA) were obtained from Beyotime Institute of Biotechnology (Shanghai, China). Liquiritin, isoliquiritin, liquiritigenin, isoliquiritigenin and glycyrrhizic acid were purchased from Chunqiu Bio-Technology Co., Ltd. (Nanjing, Jiangsu, China).
4.2. Preparation of Licorice Aqueous Extract and UHPLC-Q Extractive Orbitrap-HRMS Analysis
Licorice crude drug was soaked in ten-fold ddH2O (1:10, w/v) for 0.5 h, before being decocted for 1 h. Subsequently, the extraction was filtered through six layers of gauze, before the residue was boiled twice in a total volume of 8 and 5 times (w/v) the weight of the herbs, respectively. The filtrates were combined and concentrated using a pressure-reduced rotary evaporator at a temperature of 70 °C. The extract yield was 17.9%. The extracts were kept in a refrigerated desiccator and before each experiment, they were freshly dissolved in ddH2O to reach the desired concentrations.
The UHPLC instrument, including a Thermo Scientific Dionex Ultimate 3000 Series RS pump coupled with TCC-3000RS column compartments and a Thermo Fisher Scientific Ultimate 3000 Series WPS-3000RS autosampler controlled by Chromeleon 7.2 Software (Thermo Fisher Scientific, Waltham, MA, USA and Dionex Softron GmbH Part of Thermo Fisher Scientific, Germering, Germany), was used for analysis. Chromatographic separation was carried out at 30 °C on an Agilent poroshell 120 EC-C18 (3 mm × 100 mm, 2.7 µm). The mobile phase was delivered at a flow rate of 0.3 mL/min and consists of 0.1% formic acid-water and acetonitrile using a gradient elution as follows: 0–10 min with 5–17% B; 10–12 min with 17–17% B; 12–14 min with 17–22% B; 14–19 min with 22–22% B; 19–29 min with 22–32% B; and 29–30 min with 32–50% B; 30–34 min with 50–90% B. The full scanned data in negative was acquired at a resolving power of 70,000 FWHM at
m/
z 200. A scan range of
m/
z 100–1500 was chosen. The typical chromatogram is shown in
Figure 1, while the characterization of chemical constituents of licorice aqueous extract by UHPLC-Q Extractive Orbitrap-HRMS is presented in
Table 1.
For HPLC quantification, HPLC was performed on an Agilent HPLC 1260 system (Santa Clara, CA, USA) equipped with an auto-sampler unit, diode array detector (DAD) and a Agela venusil MP-C18 column (4.6 mm × 250 mm, 5 μm). The injection volume was 20 μL. The mobile phase was delivered at a flow rate of 1 mL/min and consists of 0.1% formic acid–water and acetonitrile using a gradient elution as follows: 0–25 min with 20–30% B; 25–35 min with 30–35% B; 35–50 min with 35–65% B; 50–60 min with 65–65% B; and 60–65 min with 20–65% B. Chromatograms were recorded at 230 nm. The column temperature was 30 °C. The following standards (Chunqiu Bio-Technology Co., Ltd., Purity: HPLC ≥ 98%) were examined: liquiritin, isoliquiritin, liquiritigenin and glycyrrhetinic acid. The data are shown in
Table 2 and the values were expressed as mean ± SD (
n = 6, 6 batches of preparations). Glycyrrhetinic acid and liquiritin were identified as the major compounds, representing 21.60 mg/g and 11.82 mg/g. The analysis was repeated consecutively using the same sample 6 times to determine precision. Additionally, the sample was analyzed at 0, 2, 4, 6, 12 and 36 h in stability tests. Six samples of licorice aqueous extract from the same batch were analyzed to measure the repeatability of this method. Relative standard deviations (RSD) of precision, stability and repeatability are shown in the following
Table 5, indicating good precision, stability and repeatability of the method.
4.3. Animals
Non-pregnant Female Imprinting Control Region (ICR) mice weighing 18–22 g (6–8 weeks old) were obtained from the Experimental Animal Center of Yangzhou University. All animals were housed in a temperature of 25 °C, humidity of 50% and light controlled (12 h light/12 h darkness) vivarium with food and water ad libitum. The animal use protocol was approved by the animal ethics committee of the School of Chinese Material Medica at China Pharmaceutical University (Approval number: SCXK (Su) 2012-0004).
4.4. Isolation of Uterus and Measurement of Uterine Contraction
Female ICR mice were pretreated with estradiol benzoate (1 mg/kg/day) by intraperitoneal injection for 3 consecutive days before the ex vivo experiments. After the animals were sacrificed by cervical dislocation, the uteri were collected to a container filled with Locke’s solution (in mmol/L: NaCl 120, KCl 4.6, CaCl2 1.5, MgSO4 1.2, KH2PO4 1.0, NaHCO3 25 and glucose 11). After removal of the adherent fat tissue and mesenteric attachments of uterine strips, the isolated uteri were individually transferred and incubated in 20 mL of Locke’s solution in organ baths at 37 °C bubbled with 95% O2 and 5% CO2. Following this, the uterus was preloaded with 1 g tension, before the amplitude of uterine contraction became stable after equilibration for 45 min. Uterine contractions were monitored by a force-displacement transducer connected to a polygraph (Model BL420E+, Tai Meng, Chengdu, China). To evaluate the influence of licorice aqueous extracts on oxytocin-stimulated uterine contractions, oxytocin (0.01 U/mL) was added to the organ bath at 15 min before different concentrations of licorice and nifedipine (positive control) were cumulatively added. The contraction was monitored for about 10 min for each concentration. The data were presented as contraction amplitude and frequency.
4.5. Effects of Oxytocin-Stimulation on Isolated Uterus Ex Vivo
The isolated uteri were randomly divided into six groups and equilibrated for 45 min in Locke’s solution at 37 °C bubbled with 95% O2 and 5% CO2. The oxytocin (0.01 U/mL) was added to the incubation solution at six time points in different groups and allowed to react. After the reaction, the uteri tissues were collected for further investigation.
4.6. Effects of Licorice Aqueous Extract on Oxytocin-Stimulated Uterus Ex Vivo
The isolated uteri were randomly divided into six groups and equilibrated for 45 min in Locke’s solution at 37 °C bubbled with 95% O2 and 5% CO2. Each uterus of the treatment groups was stimulated by oxytocin (0.01 U/mL) and a combination of licorice (0.1, 0.2 and 0.4 mg/mL) or nifedipine (0.5 ng/mL) was added. The equal volume of DMSO was added into the control and oxytocin-stimulated model group. All groups were allowed to react for 60 min. After the reaction, the uteri tissues were collected for further investigation.
4.7. Histological Tests
The uterine tissues were fixed in 10% buffered formalin and dehydrated with a graded ethanol series and embedded in paraffin. The tissues were subsequently sliced into 5-μm thick sections using a histotome. The morphological evaluation of tissues stained with hematoxylin and eosin (H & E) was performed under a light microscope (OLYMPUS DX45, Tokyo, Japan). Identification standards for evaluating the contractile status of smooth muscle cells in the uterine inner annular layer based on morphological characteristics were ascertained. The short rod-shaped cells with shorter nucleus, defined as oval nucleated cells, were in contractile status after oxytocin-stimulation. However, the normal myometrial cells were slender with round nucleus, defined as fusiform nucleated cells.
4.8. Immunofluorescence
The same tissue sections prepared from the Method 2.7 were mounted on polylysine-coated slides and deparaffinized in dimethylbenzene solution three times once for 10 min. This was followed by progressively hydrated with successive washes at room temperature in 100% ethanol, 95% ethanol in PBS, 70% ethanol in PBS and PBS. After permeabilization for 15 min in boiling citrate solution (0.1% sodium citrate, 0.1% Triton X-100 in water), the tissue slides were washed twice with PBS at room temperature. Non-specific binding sites were blocked with 3% BSA in PBS for 1 h, following which the anti-HSP27 and anti-α-SMA primary antibodies (1:100 in blocking buffer) were incubated with tissue slides overnight at 4 °C. The slides were washed three times with PBS, before the bound antibody was detected using Alexa Fluor® 647 conjugated anti-rabbit and Alexa Fluor® 488 conjugated anti-mouse secondary antibodies diluted 1:200 for 1 h at room temperature in a black wet box protected from light. The slides were washed three times with PBS, before being counterstained with DAPI dye followed by three rinses with water. The tissues were covered with anti-fade mounting medium and observed under a fluorescence microscope (DM2500, Leica Microsystems, Wetzlar, Germany). In the negative control, the primary antibody was instead replaced with PBS.
4.9. Western Blot Analysis
Uterine tissue samples were prepared by homogenization in modified radioimmunoprecipitation assay buffer (RIPA buffer: 1× PBS, 1% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 10 mg/mL PMSF, 30 μL/mL aprotinin and 100 mM sodium orthovanadate), maintained in constant agitation for 1.5 h at 4 °C and subsequently centrifuged twice at 12,000× g for 5 min at 4 °C. The supernatants were collected and the protein concentration was determined by the BCA kit. A total of 40 µg of total protein was applied to each well of a 10% SDS polyacrylamide gel and submitted to electrophoresis for 2 h at 120 V with a set of reference protein markers. Following this, the proteins were transferred onto PVDF membranes (Millipore Corp, Bedford, MA, USA) with 200 mA for 1.5 h at room temperature using a transfer buffer composed of 25 mmol/L Tris base, 192 mmol/L glycine and 20% methanol. The blots were blocked for 1 h at room temperature with a blocking buffer of 10 mmol/L Tris at a pH of 7.5, 100 mmol/L NaCl and 0.1% Tween 20. The blocking buffer was decanted and the blots were incubated with anti-HSP27, anti-pHSP27-Ser15 and anti-α-SMA primary antibodies. After washing three times with a TBS buffer composed of 10 mmol/L Tris, pH 7.5, 100 mmol/L NaCl and 0.1% Tween 20, the membrane was incubated with a peroxidase-conjugated secondary antibody depending on the source of primary antibody. The blots were finally detected with enhanced chemiluminescence reagent (ECL) and visualized using the ImageLab software 4.1 (Bio-Rad, Hercules, CA, USA). Each experiment was repeated three times in order to ensure reproducible results.
4.10. ChemGPS-NP Analysis
The principal component analysis (PCA) based global chemical space map ChemGPS-NP (
http://chemgps.bmc.uu.se) is an online tool for exploration of the regions of natural products with biologically relevant chemical space. It consists of eight principal components (dimensions, PC), derived from 35 molecular descriptors describing the physical-chemical properties. The basic interpretation of the eight dimensions of ChemGPS-NP are presented in
Table 6. The ChemGPS-NP descriptors were calculated for seven main chemical ingredients from licorice aqueous extracts (
Figure 1 and
Table 1, Compd.
1–
7) based on their structure information as simplified molecular input line entry specification (SMILES). Following this, compounds were mapped in the ChemGPS-NP global map using interpolation in terms of PCA score prediction together with a reference set of known inhibitors, targeting p38, MK2 and PKCδ separately. The source of known inhibitors for comparison was collected from online ChEMBL database and a cutoff of 10 μM in IC
50 was performed. The coordinates were plotted using SigmaPlot software (SigmaPlot 12.5, San Jose, CA, USA).
4.11. Molecular Docking
The protein crystal structures used in the docking studies were obtained from the protein data bank (PDB ID: 3R2Y for MK2; PDB ID: 1XJD for catalytic domain of PKC). The internal known inhibitors in 3R2Y and 1XJD were removed and extracted from the protein structures, re-docked into the active site to verify the docking method’s reliability (RMSD is 1.26 Å and 0.89 Å respectively, which shows the reliability of docking method). The tested compounds of SMILES format were converted to three-dimensional (3D) structures of PDB format using online SMILES translator and structure file generator (
http://cactus.nci.nih.gov/translate). Molecular docking was performed using software of Autodock4.0 (AutoDock4 and AutoDockTools4, Scripps Research Institute, MB-5 10550 N. Torrey Pines Rd., La Jolla, CA, USA). The size of the grid boxes was 40 × 40 × 40, whose center coordination were 6.502, 18.269 and 45.549 (MK2) as well as 56.374, 8.021 and 3.610 (PKC) with the spacing of 0.375 Å. The default values were used for all other parameters. The predicted protein–ligand complexes were optimized and ranked according to the empirical scoring function, which estimates the binding free energy of the ligand receptor complex.
4.12. Statistical Analysis
Results were presented as means ± SEM. Data were analyzed using GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA) by one-way ANOVA followed by Tukey’s Honestly Significant Difference test for multiple comparison testing to estimate the significance of the results. A value of p < 0.05 was considered statistically significant, while p < 0.01 was considered statistically highly significant.