Sensitive and Selective Detection of Oxo-Form Organophosphorus Pesticides Based on CdSe/ZnS Quantum Dots
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of CdSe/ZnS Core/Shell QDs
2.2. Principle of Sensitive Detection of Organophosphorus Pesticide
2.3. Optimization of Experimental Parameters
2.4. The Reproducibility of the Proposed Biosensor
2.5. The Anti-Interference Ability of the Proposed Biosensor
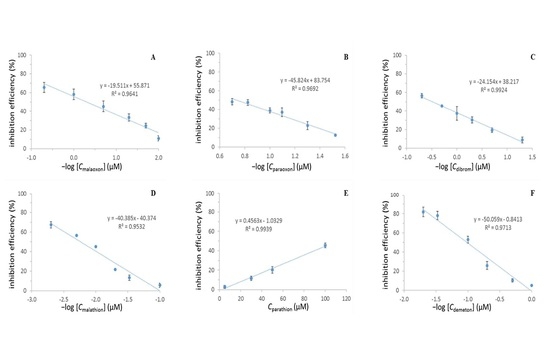
2.6. Inhibition Efficiencies of the Different Pesticides
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus and Software
3.3. Fluorescence Quenching Effect of QDs by Enzyme-Generated H2O2
3.4. Procedure for the Sensitive Determination of Pesticides in Panax ginseng
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Fantke, P.; Wieland, P.; Juraske, R.; Shaddick, G.; Sevigne-Itoiz, E.; Friedrich, R.; Jolliet, O. Parameterization models for pesticide exposure via crop consumption. Environ. Sci. Technol. 2012, 46, 12864–12872. [Google Scholar] [CrossRef] [PubMed]
- Kohler, H.R.; Triebskorn, R. Wildlife ecotoxicology of pesticides: Can we track effects to the population level and beyond? Science 2013, 341, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Fukuto, T.R. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 1990, 87, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Lopez-Fernandez, O.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gandara, J. A review on the fermentation of foods and the residues of pesticides-biotransformation of pesticides and effects on fermentation and food quality. Crit. Rev. Food Sci. 2015, 55, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.C.; Hu, J.; Cao, J.L.; Wan, J.B.; He, C.W.; Hu, Y.J.; Hu, H.; Li, P. Sensitive detection of organophosphorus pesticides in medicinal plants using ultrasound-assisted dispersive liquid-liquid microextraction combined with sweeping micellar electrokinetic chromatography. J. Agric. Food Chem. 2016, 64, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Riederer, A.M.; Hunter, R.E.; Hayden, S.W.; Ryan, P.B. Pyrethroid and organophosphorus pesticides in composite diet samples from atlanta, USA adults. Environ. Sci. Technol. 2010, 44, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Curl, C.L.; Fenske, R.A.; Kissel, J.C.; Shirai, J.H.; Moate, T.F.; Griffith, W.; Coronado, G.; Thompson, B. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ. Health Perspect. 2002, 110, A787–A792. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, S.; Valmoozi, A.A.E.; Pourayoubi, M.; Samani, K.A. Structure-activity study of phosphoramido acid esters as acetylcholinesterasf inhibitors. J. Enzym. Inhib. Med. Chem. 2008, 23, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Grimalt, S.; Dehouck, P. Review of analytical methods for the determination, of pesticide residues in grapes. J. Chromatogr. A 2016, 1433, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Kong, W.J.; Gong, B.; Miao, Q.; Qi, Y.; Yang, M.H. Rapid analysis of multi-pesticides in morinda officinalis by gc-ecd with accelerated solvent extraction assisted matrix solid phase dispersion and positive confirmation by gc-ms. J. Chromatogr. B 2015, 974, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.S.; Kong, W.J.; Wei, J.H.; Yang, M.H. Gas chromatography with flame photometric detection of 31 organophosphorus pesticide residues in alpinia oxyphylla dried fruits. Food Chem. 2014, 162, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.A.; Oturan, N.; Oturan, M.A. Electrochemically assisted remediation of pesticides in soils and water: A review. Chem. Rev. 2014, 114, 8720–8745. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Wang, J.L.; Cheng, J.; Dai, Z.F. Determination of trace copper ions with ultrahigh sensitivity and selectivity utilizing cdte quantum dots coupled with enzyme inhibition. Biosens. Bioelectron. 2012, 36, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.W.; Wei, J.F.; Ren, X.L.; Ren, J.; Tang, F.Q. A simple and sensitive fluorescence biosensor for detection of organophosphorus pesticides using H2O2-sensitive quantum dots/bi-enzyme. Biosens. Bioelectron. 2013, 47, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Mei, Q.S.; Guan, G.J.; Liu, B.H.; Wang, S.H.; Zhang, Z.P. Ligand replacement-induced fluorescence switch of quantum dots for ultrasensitive detection of organophosphorothioate pesticides. Anal. Chem. 2010, 82, 9579–9586. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Z.; Li, X.Y.; Dai, Z.F.; Liu, S.Q.; Tang, Z.Y. Detection of mixed organophosphorus pesticides in real samples using quantum dots/bi-enzyme assembly multilayers. J. Mater. Chem. 2011, 21, 16955–16962. [Google Scholar] [CrossRef]
- Zheng, Z.Z.; Zhou, Y.L.; Li, X.Y.; Liu, S.Q.; Tang, Z.Y. Highly-sensitive organophosphorous pesticide biosensors based on nanostructured films of acetylcholinesterase and cdte quantum dots. Biosens. Bioelectron. 2011, 26, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hao, T.F.; Xu, Y.Q.; Lu, K.; Li, H.J.; Yan, Y.S.; Zhou, Z.P. Facile polymerizable surfactant inspired synthesis of fluorescent molecularly imprinted composite sensor via aqueous cdte quantum dots for highly selective detection of lambda-cyhalothrin. Sens. Actuators B Chem. 2016, 224, 315–324. [Google Scholar] [CrossRef]
- Ban, R.; Zhu, J.J.; Zhang, J.R. Manganese-doped zns quantum dots as a phosphorescent probe for use in the bi-enzymatic determination of organophosphorus pesticides. Microchim. Acta 2014, 181, 1591–1599. [Google Scholar] [CrossRef]
- Steiner, M.S.; Duerkop, A.; Wolfbeis, O.S. Optical methods for sensing glucose. Chem. Soc. Rev. 2011, 40, 4805–4839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Cheng, F.F.; Li, J.J.; Zhu, J.J.; Lu, Y. Fluorescent nanoprobes for sensing and imaging of metal ions: Recent advances and future perspectives. Nano Today 2016, 11, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Zheng, T.T.; Cheng, F.F.; Zhang, J.R.; Zhu, J.J. Toward the early evaluation of therapeutic effects: An electrochemical platform for ultrasensitive detection of apoptotic cells. Anal. Chem. 2011, 83, 7902–7909. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, P. The use of nanocrystals in biological detection. Nat. Biotechnol. 2004, 22, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Mongin, C.; Garakyaraghi, S.; Razgoniaeva, N.; Zamkov, M.; Castellano, F.N. Direct observation of triplet energy transfer from semiconductor nanocrystals. Science 2016, 351, 369–372. [Google Scholar] [CrossRef]
- Lia, J.J.; Zhu, J.J. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst 2013, 138, 2506–2515. [Google Scholar] [CrossRef]
- Danek, M.; Jensen, K.F.; Murray, C.B.; Bawendi, M.G. Synthesis of luminescent thin-film cdse/znse quantum dot composites using cdse quantum dots passivated with an overlayer of znse. Chem. Mater. 1996, 8, 173–180. [Google Scholar] [CrossRef]
- Hines, M.A.; Guyot-Sionnest, P. Synthesis and characterization of strongly luminescing zns-capped cdse nanocrystals. J. Phys. Chem. 1996, 100, 468–471. [Google Scholar] [CrossRef]
- Pichaandi, J.; van Veggel, F.C.J.M. Near-infrared emitting quantum dots: Recent progress on their synthesis and characterization. Coord. Chem. Rev. 2014, 263, 138–150. [Google Scholar] [CrossRef]
- Zrazhevskiy, P.; Sena, M.; Gao, X.H. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem. Soc. Rev. 2010, 39, 4326–4354. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.J.; Zheng, J.Y.; Xu, J.M.; Rastogi, V.K.; Cheng, T.C.; DeFrank, J.J.; Leblanc, R.M. (CdSe)ZnS quantum dots and organophosphorus hydrolase bioconjugate as biosensors for detection of paraoxon. J. Phys. Chem. B 2005, 109, 3793–3799. [Google Scholar] [CrossRef]
- Du, D.; Chen, A.Q.; Xie, Y.Y.; Zhang, A.D.; Lin, Y.H. Nanoparticle-based immunosensor with apoferritin templated metallic phosphate label for quantification of phosphorylated acetylcholinesterase. Biosens. Bioelectron. 2011, 26, 3857–3863. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.D.; Wang, J.; Barry, R.; Petersen, C.; Timchalk, C.; Gassman, P.L.; Lin, Y.H. Nanoparticle-based electrochemical immunosensor for the detection of phosphorylated acetylcholinesterase: An exposure biomarker of organophosphate pesticides and nerve agents. Chem. Eur. J. 2008, 14, 9951–9959. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.T.; Deng, J.J.; Zhang, M.; Shi, G.Y.; Zhou, T.S. Quantum dot-DNA aptamer conjugates coupled with capillary electrophoresis: A universal strategy for ratiometric detection of organophosphorus pesticides. Talanta 2016, 146, 55–61. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed; European Commission Document; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Yi, Y.H.; Zhu, G.B.; Liu, C.; Huang, Y.; Zhang, Y.Y.; Li, H.T.; Zhao, J.N.; Yao, S.Z. A label-free silicon quantum dots-based photoluminescence sensor for ultrasensitive detection of pesticides. Anal. Chem. 2013, 85, 11464–11470. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Ni, Y.N.; Kokot, S. Unmodified silver nanoparticles for rapid analysis of the organophosphorus pesticide, dipterex, often found in different waters. Sens. Actuators B Chem. 2014, 193, 205–211. [Google Scholar] [CrossRef]
- Chen, L.N.; Song, F.R.; Liu, Z.Q.; Zheng, Z.; Xing, J.P.; Liu, S.Y. Multi-residue method for fast determination of pesticide residues in plants used in traditional chinese medicine by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2012, 1225, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, H.Y.; Ma, S.C.; Lu, J.; Lin, R.C. Determination of 195 pesticide residues in chinese herbs by gas chromatography-mass spectrometry using analyte protectants. J. Chromatogr. A 2011, 1218, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Homem, V.; Moreira, J.L.; Madeira, L.M.; Alves, A. Optimisation and application of dispersive liquid-liquid microextraction for simultaneous determination of carbamates and organophosphorus pesticides in waters. Anal. Methods UK 2013, 5, 2736–2745. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds include pesticide standards and ginseng samples are available from the authors. |





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Cao, J.; Hu, H.; Yang, Q.; Yang, F.; Wan, J.; Su, H.; He, C.; Li, P.; Wang, Y. Sensitive and Selective Detection of Oxo-Form Organophosphorus Pesticides Based on CdSe/ZnS Quantum Dots. Molecules 2017, 22, 1421. https://doi.org/10.3390/molecules22091421
Wei J, Cao J, Hu H, Yang Q, Yang F, Wan J, Su H, He C, Li P, Wang Y. Sensitive and Selective Detection of Oxo-Form Organophosphorus Pesticides Based on CdSe/ZnS Quantum Dots. Molecules. 2017; 22(9):1421. https://doi.org/10.3390/molecules22091421
Chicago/Turabian StyleWei, Jinchao, Jiliang Cao, Hao Hu, Qing Yang, Fengqing Yang, Jianbo Wan, Huanxing Su, Chengwei He, Peng Li, and Yitao Wang. 2017. "Sensitive and Selective Detection of Oxo-Form Organophosphorus Pesticides Based on CdSe/ZnS Quantum Dots" Molecules 22, no. 9: 1421. https://doi.org/10.3390/molecules22091421
APA StyleWei, J., Cao, J., Hu, H., Yang, Q., Yang, F., Wan, J., Su, H., He, C., Li, P., & Wang, Y. (2017). Sensitive and Selective Detection of Oxo-Form Organophosphorus Pesticides Based on CdSe/ZnS Quantum Dots. Molecules, 22(9), 1421. https://doi.org/10.3390/molecules22091421