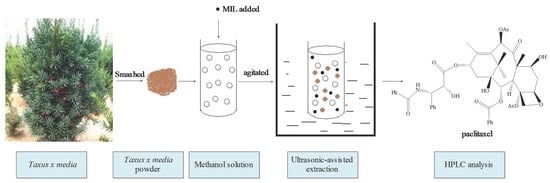
Ultrasonic Assisted Extraction of Paclitaxel from Taxus x media Using Ionic Liquids as Adjuvants: Optimization of the Process by Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of the Optimal IL as Adjuvant
2.2. Effect of IL Amount
2.3. Effect of Methanol Concentration
2.4. Effect of Solid–Liquid Ratio
2.5. Effect of Extraction Temperature
2.6. Effect of Ultrasonic Irradiation Time
2.7. Optimization of the Extraction by RSM
3. Materials and Methods
3.1. Materials and Reagents
3.2. Solvent Extraction of Paclitaxel
3.3. HPLC Conditions
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taura, K.; Yamamoto, S.; Hayashi, S.; Shioya, S. Enhanced paclitaxel production by addition of water-soluble 5-aminolevulinic acid and in situ extraction with lauryl alcohol in a suspension callus culture. Solvent Extr. Res. Dev. 2013, 20, 65–70. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.H. Effect of water content of organic solvent on microwave-assisted extraction efficiency of paclitaxel from plant cell culture. Korean J. Chem. Eng. 2011, 28, 1561–1565. [Google Scholar] [CrossRef]
- Zu, Y.; Wang, Y.; Fu, Y.; Li, S.; Sun, R.; Liu, W.; Luo, H. Enzyme-assisted extraction of paclitaxel and related taxanes from needles of taxuschinensis. Sep. Purif. Technol. 2009, 68, 238–243. [Google Scholar] [CrossRef]
- Wu, J.; Lin, L. Enhancement of taxol production and release in taxuschinensis cell cultures by ultrasound, methyl jasmonate and in situ solvent extraction. Appl. Microbiol. Biot. 2003, 62, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Luo, L.; Bi, P.; Zheng, Y.; Zhao, J. Determination of taxol, cephalomannine and 7-epi-taxol in taxus by prp-6 solid phase extraction and high performance liquid chromatography. Anal. Lett. 2005, 38, 929–937. [Google Scholar] [CrossRef]
- Ketchum, R.E.B.; Luong, J.V.; Gibson, D.M. Efficient extraction of paclitaxel and related taxoids from leaf tissue of taxus using a potable solvent system. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 1715–1732. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Willauer, H.D.; Swatloski, R.P.; Visser, A.E.; Rogers, R.D. Room temperature ionic liquids as novel media for “Clean” Liquid-liquid extraction. Chem. Commun. 1998, 1765–1766. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic liquids-solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.D.; Zhang, C.; Hantao, L.W.; Anderson, J.L. Ionic liquids in analytical chemistry: Fundamentals, advances, and perspectives. Anal. Chem. 2013, 86, 262–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G. Functionalized imidazolium salts for task-specific ionic liquids and their applications. Chem. Commun. 2006, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Giernoth, R. Task-specific ionic liquids. Angew. Chem. Int. Ed. 2010, 49, 2834–2839. [Google Scholar] [CrossRef] [PubMed]
- Claudio, A.F.M.; Ferreira, A.M.; Freire, M.G.; Coutinho, J.A.P. Enhanced extraction of caffeine from guarana seeds using aqueous solutions of ionic liquids. Green Chem. 2013, 15, 2002–2010. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Li, Y.; Chi, R. Optimization of ionic liquid-based microwave-assisted extraction of isoflavones from radix puerariae by response surface methodology. Sep. Purif. Technol. 2014, 135, 285. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, Z.; Li, F.; Li, X. An effective method for the extraction and purification of chlorogenic acid from ramie (boehmerianivea l.) leaves using acidic ionic liquids. Ind. Crop Prod. 2016, 89, 78–86. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Q.; Mo, K.; Fei, S.; Gu, H.; Yang, L. Optimization of ionic liquid-based homogenate extraction of orientin and vitexin from the flowers of trolliuschinensis and its application on a pilot scale. Sep. Purif. Technol. 2017, 175, 147–157. [Google Scholar] [CrossRef]
- Tan, T.; Lai, C.J.S.; OuYang, H.; He, M.Z.; Feng, Y. Ionic liquid-based ultrasound-assisted extraction and aqueous two-phase system for analysis of caffeoylquinic acids from flosloniceraejaponicae. J. Pharmaceut. Biomed. 2016, 120, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Bubalo, M.C.; Radosevic, K.; Redovnikovic, I.R.; Halambek, J.; Srcek, V.G. A brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol. Environ. Safe 2014, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Row, K.H. Comparison of ionic liquids and deep eutectic solvents as additives for the ultrasonic extraction of astaxanthin from marine plants. J. Ind. Eng. Chem. 2016, 39, 87–92. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Faustino, V.F.M.; Mondal, D.; Coutinho, J.A.P.; Freire, M.G. Improving the extraction and purification of immunoglobulin g by the use of ionic liquids as adjuvants in aqueous biphasic systems. J. Biotechnol. 2016, 236, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, Q.; Wu, S.; Sun, D.; Tan, Z. Salting-out extraction of sinomenine from sinomeniumacutum by an alcohol/salt aqueous two-phase system using ionic liquids as additives. J. Chem. Technol. Biot. 2017. [Google Scholar] [CrossRef]
- Wang, J.; Yao, H.; Nie, Y.; Bai, L.; Zhang, X.; Li, J. Application of iron-containing magnetic ionic liquids in extraction process of coal direct liquefaction residues. Ind. Eng. Chem. Res. 2012, 51, 3776–3782. [Google Scholar] [CrossRef]
- Wang, J.; Yao, H.; Nie, Y.; Zhang, X.; Li, J. Synthesis and characterization of the iron-containing magnetic ionic liquids. J. Mol. Liq. 2012, 169, 152–155. [Google Scholar] [CrossRef]
- Santos, E.; Albo, J.; Irabien, A. Magnetic ionic liquids: Synthesis, properties and applications. RSC Adv. 2014, 4, 40008–40018. [Google Scholar] [CrossRef]
- Deng, N.; Li, M.; Zhao, L.; Lu, C.; De Rooy, S.L.; Warner, I.M. Highly efficient extraction of phenolic compounds by use of magnetic room temperature ionic liquids for environmental remediation. J. Hazard. Mater. 2011, 192, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Xu, B.; Li, X.; Jin, R.; Zhang, H.; Song, D. Magnetic ionic liquid-based dispersive liquid-liquid microextraction for the determination of triazine herbicides in vegetable oils by liquid chromatography. J. Chromatogr. A 2014, 1373, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Yao, S. Magnetic ionic liquid aqueous two-phase system coupled with high performance liquid chromatography: A rapid approach for determination of chloramphenicol in water environment. J. Chromatogr. A 2017, 1481, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Albo, J.; Santos, E.; Neves, L.A.; Simeonov, S.P.; Afonso, C.A.M.; Crespo, J.G.; Irabien, A. Separation performance of co2 through supported magnetic ionic liquid membranes (smilms). Sep. Purif. Technol. 2012, 97, 26–33. [Google Scholar] [CrossRef]
- Bogdanov, M.G. Ionic liquids as alternative solvents for extraction of natural products. In Alternative Solvents for Natural Products Extraction; Chemat, F., Abert-Vian, M., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 2014; pp. 127–166. [Google Scholar]
- McPartland, T.J.; Patil, R.A.; Malone, M.F.; Roberts, S.C. Liquid-liquid extraction for recovery of paclitaxel from plant cell culture: Solvent evaluation and use of extractants for partitioning and selectivity. Biotechnol. Prog. 2012, 28, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Son, J.S.; Na, G.H.; Hong, S.S.; Park, Y.S.; Song, J.Y. Mass production of paclitaxel by plant cell culture. J. Plant Biotechnol. 2002, 29, 59–62. [Google Scholar] [CrossRef]
- Kim, G.J.; Kim, J.H. A simultaneous microwave-assisted extraction and adsorbent treatment process under acidic conditions for recovery and separation of paclitaxel from plant cell cultures. Korean J. Chem. Eng. 2015, 32, 1023–1028. [Google Scholar] [CrossRef]
- Kawamura, F.; Kikuchi, Y.; Ohira, T.; Yatagai, M. Accelerated solvent extraction of paclitaxel and related compounds from the bark of taxuscuspidata. J. Nat. Prod. 1999, 62, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Kim, J.H. Enhancement of extraction efficiency of paclitaxel from biomass using ionic liquid-methanol co-solvents under acidic conditions. Process Biochem. 2015, 50, 989–996. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Y.; Han, M.; Yang, L. Aqueous ionic liquid based ultrasonic assisted extraction of eight ginsenosides from ginseng root. Ultrason. Sonochem. 2013, 20, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Ha, G.S.; Kim, J.H. Kinetic and thermodynamic characteristics of ultrasound-assisted extraction for recovery of paclitaxel from biomass. Process Biochem. 2016, 51, 1664–1673. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, J.H. Kinetics and thermodynamics of paclitaxel extraction from plant cell culture. Korean J. Chem. Eng. 2016, 33, 3175–3183. [Google Scholar] [CrossRef]
- Fan, J.P.; Cao, J.; Zhang, X.H.; Huang, J.Z.; Kong, T.; Tong, S.; Tian, Z.Y.; Xie, Y.L.; Xu, R.; Zhu, J.H. Optimization of ionic liquid based ultrasonic assisted extraction of puerarin from radix puerariaelobatae by response surface methodology. Food Chem. 2012, 135, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds paclitaxel from Taxus x media leaves are available from the authors. |





| Runs | Factor A: IL Amount (wt %) | Factor B: Solid–Liquid Ratio | Factor C: Ultrasonic Irradiation Time(min) | Extraction Yield (mg/g) |
|---|---|---|---|---|
| 1 | 0.5 | 1:5 | 30 | 0.129 |
| 2 | 1.5 | 1:5 | 30 | 0.176 |
| 3 | 0.5 | 1:15 | 30 | 0.145 |
| 4 | 1.5 | 1:15 | 30 | 0.186 |
| 5 | 0.5 | 1:10 | 20 | 0.152 |
| 6 | 1.5 | 1:10 | 20 | 0.198 |
| 7 | 0.5 | 1:10 | 40 | 0.156 |
| 8 | 1.5 | 1:10 | 40 | 0.201 |
| 9 | 1.0 | 1:5 | 20 | 0.172 |
| 10 | 1.0 | 1:15 | 20 | 0.183 |
| 11 | 1.0 | 1:5 | 40 | 0.165 |
| 12 | 1.0 | 1:15 | 40 | 0.186 |
| 13 | 1.0 | 1:10 | 30 | 0.229 |
| 14 | 1.0 | 1:10 | 30 | 0.224 |
| 15 | 1.0 | 1:10 | 30 | 0.219 |
| 16 | 1.0 | 1:10 | 30 | 0.227 |
| 17 | 1.0 | 1:10 | 30 | 0.226 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 1.60 × 10−2 | 9 | 1.742 × 10−3 | 154.83 | <0.0001 |
| A | 4.005 × 10−3 | 1 | 4.005 × 10−3 | 356.01 | <0.0001 |
| B | 4.205 × 10−4 | 1 | 4.205 × 10−4 | 37.38 | 0.0005 |
| C | 1.125 × 10−6 | 1 | 1.125 × 10−6 | 0.10 | 0.7611 |
| AB | 9.00 × 10−6 | 1 | 9.00 × 10−6 | 0.80 | 0.4008 |
| AC | 2.50 × 10−7 | 1 | 2.50 × 10−7 | 0.022 | 0.8857 |
| BC | 2.50 × 10−5 | 1 | 2.50 × 10−5 | 2.22 | 0.1797 |
| A2 | 4.551 × 10−3 | 1 | 4.551 × 10−3 | 404.50 | <0.0001 |
| B2 | 4.62 × 10−3 | 1 | 4.62 × 10−3 | 410.67 | <0.0001 |
| C2 | 9.953 × 10−4 | 1 | 9.953 × 10−4 | 88.47 | <0.0001 |
| Residual | 7.875 × 10−5 | 7 | 1.125 × 10−5 | ||
| Lack of Fit | 2.075 × 10−5 | 3 | 6.917 × 10−6 | 0.48 | 0.7153 |
| Pure Error | 5.8 × 10−5 | 4 | 1.45 × 10−5 | ||
| Cor Total | 1.61 × 10−2 | 16 |
| Abbreviations of ILs (Full Names) | Cations | Anions |
|---|---|---|
| [APMIM]Cl (1-aminopropyl-3-methylimidazolium chloride) |  | Cl- |
| [C2OHMIM]Cl (1-ethoxyl-3-methyl-imidazolium chloride) |  | Cl- |
| [C2MIM]Br (1-ethyl-3-methyl-imidazolium bromide) |  | Br- |
| [C2OHMIM]FeCl4 (1-ethoxyl-3-methyl-imidazolium tetrachloroferrate) |  | FeCl4- |
| [C2MIM]FeCl3Br (1-ethyl-3-methyl-imidazolium bromotrichloroferrate) |  | FeCl3Br- |
| [C4MIM]FeCl3Br (1-butyl-3-methyl-imidazolium bromotrichloroferrate) |  | FeCl3Br- |
| [C6MIM]FeCl3Br (1-hexyl-3-methyl-imidazolium bromotrichloroferrate) |  | FeCl3Br- |
| [C8MIM]FeCl3Br(1-octyl-3-methyl-imidazolium bromotrichloroferrate) |  | FeCl3Br- |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Li, Q.; Wang, C.; Zhou, W.; Yang, Y.; Wang, H.; Yi, Y.; Li, F. Ultrasonic Assisted Extraction of Paclitaxel from Taxus x media Using Ionic Liquids as Adjuvants: Optimization of the Process by Response Surface Methodology. Molecules 2017, 22, 1483. https://doi.org/10.3390/molecules22091483
Tan Z, Li Q, Wang C, Zhou W, Yang Y, Wang H, Yi Y, Li F. Ultrasonic Assisted Extraction of Paclitaxel from Taxus x media Using Ionic Liquids as Adjuvants: Optimization of the Process by Response Surface Methodology. Molecules. 2017; 22(9):1483. https://doi.org/10.3390/molecules22091483
Chicago/Turabian StyleTan, Zhijian, Qiao Li, Chaoyun Wang, Wanlai Zhou, Yuanru Yang, Hongying Wang, Yongjian Yi, and Fenfang Li. 2017. "Ultrasonic Assisted Extraction of Paclitaxel from Taxus x media Using Ionic Liquids as Adjuvants: Optimization of the Process by Response Surface Methodology" Molecules 22, no. 9: 1483. https://doi.org/10.3390/molecules22091483
APA StyleTan, Z., Li, Q., Wang, C., Zhou, W., Yang, Y., Wang, H., Yi, Y., & Li, F. (2017). Ultrasonic Assisted Extraction of Paclitaxel from Taxus x media Using Ionic Liquids as Adjuvants: Optimization of the Process by Response Surface Methodology. Molecules, 22(9), 1483. https://doi.org/10.3390/molecules22091483