Synthesis and Antifungal Activity of Novel 3-Caren-5-One Oxime Esters
Abstract
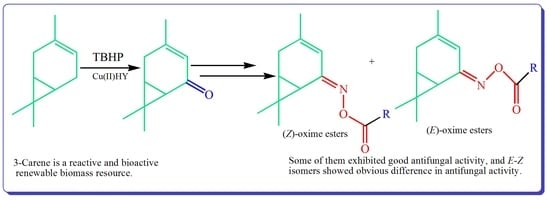
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Antifungal Activity
3. Experimental Section
3.1. General Information
3.2. Preparation of Catalyst
3.3. Synthesis of 3-Caren-5-One (2)
3.4. Synthesis of 3-Caren-5-One Oxime (3)
3.5. General Procedure for Synthesis of 3-Caren-5-One Oxime Esters (Z)-4a–4w, (E)-4f′, 4l′, 4r′
3.6. Antifungal Activity Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Qiu, C.L.; Smuts, J.; Schug, K.A. Analysis of terpenes and turpentines using gas chromatography with vacuum ultraviolet detection. J. Sep. Sci. 2017, 40, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Tissandie, L.; Filippi, J.J.; Tomi, F. New pinane derivatives found in essential oils of Calocedrus decurrens. Molecules 2017, 22, 921. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Rout, P.K.; Misra, L.N.; Gupta, P.; Singh, N.; Darokar, M.P.; Saikia, D.; Singh, S.C.; Bhakuni, R.S. Chemical composition and bioactivity of Boswellia serrata Roxb. essential oil in relation to geographical variation. Plant Biosyst. 2017, 151, 623–629. [Google Scholar] [CrossRef]
- Ibrahim, T.A.; El-Hela, A.A.; El-Hefnawy, H.M.; Al-Taweel, A.M.; Perveen, S. Chemical composition and antimicrobial activities of essential oils of some coniferous plants cultivated in Egypt. Iran. J. Pharm. Res. 2017, 16, 328–337. [Google Scholar] [PubMed]
- Li, Z.; Wang, M.; Peng, L. Chemical composition analysis of essential oil from Mosla chinensis maxim. cv. Jiangxiangru and inhibitory activity of the oil and its major constituents on biofilm formation of Staphylococcus aureus. Food Sci. 2016, 37, 138–143. [Google Scholar]
- Smeriglio, A.; Denaro, M.; Barreca, D.; Calderaro, A.; Bisignano, C.; Ginestra, G.; Bellocco, E.; Trombetta, D. In vitro evaluation of the antioxidant, cytoprotective, and antimicrobial properties of essential oil from Pistacia vera L. Variety Bronte Hull. Int. J. Mol. Sci. 2017, 18, 1212. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Lin, J.; Han, B. Evaluation of attraction of volatile semiochemicals from tea shoots to the mymarid Stethynium empoascae. Acta Ecol. Sin. 2016, 36, 3785–3795. [Google Scholar]
- Gray, C.A.; Runyon, J.B.; Jenkins, M.J.; Giunta, A.D. Mountain pine beetles use volatile cues to locate host limber pine and avoid non-host great basin bristlecone pine. PLoS ONE 2015, 10, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Wajs-Bonikowska, A.; Stobiecka, A.; Bonikowski, R.; Krajewska, A.; Sikora, M.; Kula, J. A comparative study on composition and antioxidant activities of supercritical carbon dioxide, hexane and ethanol extracts from blackberry (Rubus fruticosus) growing in Poland. J. Sci. Food Agric. 2017, 97, 3576–3583. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, N.; Chen, H.; Zhong, B.; Yang, A.; Kuang, F.; Ouyang, Z.; Chun, J. Fumigant activity of sweet orange essential oil fractions against red imported fire ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2017, 4, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Bolli, M.H.; Muller, C.; Mathys, B.; Abele, S.; Birker, M.; Bravo, R.; Bur, D.; Hess, P.; Kohl, C.; Lehmann, D.; et al. Novel S1P1 receptor agonists—Part 1: From pyrazoles to thiophenes. J. Med. Chem. 2013, 56, 9737–9755. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yeh, C.H.; Kuttruff, C.A.; Jorgensen, L.; Dunstl, G.; Felding, J.; Natarajan, S.R.; Baran, P.S. C-H oxidation of ingenanes enables potent and selective protein kinase C isoform activation. Angew. Chem. Int. Ed. 2015, 54, 14044–14048. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, S.; Chu, H.; Felding, J.; Baran, P.S. Nineteen-step total synthesis of (+)-phorbol. Nature 2016, 532, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Grue-Sorensen, G.; Mansson, K.; Vedso, P.; Soor, A.; Stahlhut, M.; Bertelsen, M.; Engell, K.M.; Hogberg, T. Syntheses, biological evaluation and SAR of ingenol mebutate analogues for treatment of actinic keratosis and non-melanoma skin cancer. Bioorg. Med. Chem. Lett. 2013, 23, 5624–5629. [Google Scholar] [CrossRef] [PubMed]
- McKerrall, S.J.; Joergensen, L.; Kuttruff, C.A.; Ungeheuer, F.; Baran, P.S. Development of a concise synthesis of (−)-ingenol. J. Am. Chem. Soc. 2014, 136, 5799–5810. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Li, H.; Jin, Z.C.; Liu, W.Y.; Xiao, Y.; He, H.; Wang, Q.M.; Shi, Y.J. Synthesis and bioactivity of novel 1-methyl-3-trifluoromethyl-5-subsituent-1H-pyrazole-4-carbaldehyde-O-(4-trifluoromethylbenzoyl)oximes. Chin. J. Org. Chem. 2016, 36, 185–190. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.B.; Li, S.C.; Ma, J.C.; Lv, M.; Xu, H. Semisynthesis of ssters of fraxinellone C4/10-oxime and their pesticidal activities. J. Agric. Food Chem. 2016, 64, 5472–5478. [Google Scholar] [CrossRef] [PubMed]
- Li, T.G.; Liu, J.P.; Han, J.T.; Fu, B.; Wang, D.Q.; Wang, M.G. Synthesis and herbicidal activity of alpha-phenylsulfonylcyclododecanone oxime esters. Chin. J. Org. Chem. 2009, 29, 898–903. [Google Scholar]
- Wang, X.; Xia, L.; Xie, Y.; Wang, X.; Xiao, W.; Zhong, X.; Huang, M.; Xue, W. Synthesis and antiviral activities of curcumin derivatives bearing oxime esters moiety. Agrochemicals 2016, 55, 641–646. [Google Scholar]
- Chen, H.P.; Zhao, Z.Z.; Li, Z.H.; Dong, Z.J.; Wei, K.; Bai, X.; Zhang, L.; Wen, C.N.; Feng, T.; Liu, J.K. Novel natural oximes and oxime esters with a vibralactone backbone from the Basidiomycete Boreostereum vibrans. ChemistryOpen 2016, 5, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Shen, F.J.; Feng, G.L.; Yuan, R.X. Synthesis and anticancer activity of 4-azasteroidal-20-oxime derivatives. J. Chem. Res. 2015, 39, 527–530. [Google Scholar] [CrossRef]
- Hamid, A.A.; Kaushal, T.; Ashraf, R.; Singh, A.; Gupta, A.C.; Prakash, O.; Sarkar, J.; Chanda, D.; Bawankule, D.U.; Khan, F.; et al. (22β,25R)-3β-Hydroxy-spirost-5-en-7-iminoxy-heptanoic acid exhibits anti-prostate cancer activity through caspase pathway. Steroids 2017, 119, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, H.B.; Yusufoglu, A.S.; Acik, L.; Aydin, B.; Arslan, L. Synthesis, (E)/(Z)-isomerization, and DNA binding, antibacterial, and antifungal activities of novel oximes and O-substituted oxime ethers. Turk. J. Chem. 2016, 40, 816–829. [Google Scholar] [CrossRef]
- Krishnan, K.G.; Sivakumar, R.; Thanikachalam, V. Synthesis, structural characterization and antimicrobial evaluation of some novel piperidin-4-one oxime esters. J. Serb. Chem. Soc. 2015, 80, 1101–1111. [Google Scholar] [CrossRef]
- Sammaiah, A.; Kaki, S.S.; Manoj, G.; Poornachandra, Y.; Kumar, C.G.; Prasad, R.B.N. Novel fatty acid esters of apocynin oxime exhibit antimicrobial and antioxidant activities. Eur. J. Lipid Sci. Technol. 2015, 117, 692–700. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Singh, R.; Sati, O.P.; Walia, S.; Garg, N. Synthesis and antimicrobial activity of esters of 3-ethoxy-4-hydroxybenzaldehyde oxime. Toxicol. Environ. Chem. 2017, 99, 1–9. [Google Scholar] [CrossRef]
- Lin, G.S.; Duan, W.G.; Yang, L.X.; Huang, M.; Lei, F.H. Synthesis and antifungal activity of novel myrtenal-based 4-methyl-1,2,4-triazole-thioethers. Molecules 2017, 22, 193. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Wang, X.; Duan, W.G.; Lin, G.S. Synthesis and in vitro anticancer activity of novel dehydroabietic acid-based acylhydrazones. Molecules 2017, 22, 1087. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Duan, W.; Lin, G.; Liu, L.; Zhang, R.; Li, D. Synthesis and antifungal activity of dehydroabietic acid-based 1,3,4-thiadiazole-thiazolidinone compounds. Mol. Divers. 2016, 20, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Li, F.Y.; Duan, W.G.; Liao, J.N.; Lin, Z.D.; Lin, G.S.; Cen, B.; Lei, F.H. Synthesis and antifungal activity of camphoric acid-based acylhydrazone compounds. Holzforschung 2014, 68, 889–895. [Google Scholar] [CrossRef]
- Li, F.Y.; Mo, Q.J.; Duan, W.G.; Lin, G.S.; Cen, B.; Chen, N.Y.; Yang, Z.Q. Synthesis and insecticidal activities of N-(5-dehydroabietyl-1,3,4-thiadiazol-2-yl)-benzenesulfonamides. Med. Chem. Res. 2014, 23, 4420–4426. [Google Scholar] [CrossRef]
- Lin, G.S.; Ma, C.H.; Duan, W.G.; Cen, B.; Lei, F.H.; Yang, Z.Q. Synthesis and biological activities of α-pinene-based dithiadiazoles. Holzforschung 2014, 68, 75–83. [Google Scholar] [CrossRef]
- Huang, D.Y.; Duan, W.G.; Lin, G.S.; Bai, X.; Xiao, H.; Yang, Z.Q. Synthesis and antifungal activities of 2-sustituted acylamino-5-(α-campholenicaldehyde)-based-1,3,4-thiadiazole compounds. Chem. Ind. For. Prod. 2016, 36, 61–69. [Google Scholar]
- Alfayate, A.; Márquez-Álvarez, C.; Grande-Casas, M.; Bernardo-Maestro, B.; Sánchez-Sánchez, M.; Pérez-Pariente, J. Enhanced catalytic activity of TAPO-5 in the oxidation of cyclohexene with hydrogen peroxide under anhydrous conditions. Catal. Today 2013, 213, 211–218. [Google Scholar] [CrossRef]
- Vanelderen, P.; Snyder, B.E.; Tsai, M.L.; Hadt, R.G.; Vancauwenbergh, J.; Coussens, O.; Schoonheydt, R.A.; Sels, B.F.; Solomon, E.I. Spectroscopic definition of the copper active sites in mordenite: Selective methane oxidation. J. Am. Chem. Soc. 2015, 137, 6383–6392. [Google Scholar] [CrossRef] [PubMed]
- Narsimhan, K.; Iyoki, K.; Dinh, K.; Roman-Leshkov, Y. Catalytic oxidation of methane into methanol over copper-exchanged zeolites with oxygen at low temperature. ACS Cent. Sci. 2016, 2, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Chanquía, C.M.; Cánepa, A.L.; Bazán-Aguirre, J.; Sapag, K.; Rodríguez-Castellón, E.; Reyes, P.; Herrero, E.R.; Casuscelli, S.G.; Eimer, G.A. Copper-containing spherical mesoporous silicates prepared by template-ion exchange: A multitechnique characterization and oxidation properties. Microporous Mesoporous Mater. 2012, 151, 2–12. [Google Scholar] [CrossRef]
- Krishnan, K.G.; Sivakumar, R.; Thanikachalam, V. Synthesis and spectral study of some novel oxime esters derived from 3-azabicyclo 3.3.1 nonan-9-one oxime. Lett. Org. Chem. 2015, 12, 31–37. [Google Scholar] [CrossRef]
- Fan, M.G.; Fang, J.L.; Zhou, L.C.; Li, W.L.; Li, B.; Xing, J.M.; Liu, Z.L. Distribution of the Cu ions in the CuHY zeolite and its performance of desulfurization. Chem. J. Chin. Univ. 2008, 29, 1834–1840. [Google Scholar]
- Su, N.N.; Li, Y.; Yu, S.J.; Zhang, X.; Liu, X.H.; Zhao, W.G. Microwave-assisted synthesis of some novel 1,2,3-triazoles by click chemistry, and their biological activity. Res. Chem. Intermed. 2012, 39, 759–766. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2, (Z)-3a, (E)-3b, (Z)-4a–4w, and (E)-4f′, 4l′, 4r′ are available from the authors. |



| Compounds | Relative Inhibition Rate (%) against the Tested Fungi | |||||||
|---|---|---|---|---|---|---|---|---|
| F. oxysporum f. sp. cucumerinum | C. arachidicola | P. piricola | A. solani | G. zeae | R. solani | B. myadis | C. orbicalare | |
| (Z)-4a (R = Ph) | 28.8 | 35.0 | 69.4 | 13.7 | 21.8 | 0 | 32.2 | 43.3 |
| (Z)-4b (R = o-ClPh) | 16.3 | 30.0 | 0 | 0 | 21.8 | 18.3 | 23.9 | 30.5 |
| (Z)-4c (R = m-ClPh) | 22.5 | 0 | 44.4 | 0 | 21.8 | 17.1 | 26.7 | 33.1 |
| (Z)-4d (R = p-ClPh) | 28.8 | 0 | 28.8 | 0 | 19.8 | 0 | 29.4 | 33.1 |
| (Z)-4e (R = o-FPh) | 31.9 | 45.0 | 41.3 | 35.9 | 37.5 | 35.0 | 26.7 | 45.9 |
| (Z)-4f (R = p-FPh) | 25.6 | 15.0 | 30.7 | 0.0 | 15.9 | 0 | 26.7 | 23.8 |
| (Z)-4g (R = o-OMePh) | 25.6 | 0 | 60.0 | 21.1 | 13.9 | 0 | 26.7 | 35.6 |
| (Z)-4h (R = o-MePh) | 31.9 | 50.0 | 28.8 | 21.1 | 29.6 | 38.6 | 29.4 | 43.3 |
| (Z)-4i (R = m-MePh) | 19.4 | 15.0 | 85.0 | 0 | 12.0 | 0 | 23.9 | 0 |
| (Z)-4j (R = p-MePh) | 38.1 | 20.0 | 81.9 | 24.8 | 17.8 | 0 | 35.0 | 53.6 |
| (Z)-4k (R = 2,4-ClPh) | 28.8 | 55.0 | 16.3 | 13.7 | 17.8 | 18.3 | 23.9 | 53.6 |
| (Z)-4l (R = 2,3-ClPh) | 25.6 | 15.0 | 28.8 | 17.4 | 19.8 | 0 | 21.1 | 43.3 |
| (Z)-4m (R = p-CH2ClPh) | 56.9 | 20.0 | 41.3 | 0 | 19.8 | 0 | 21.1 | 40.8 |
| (Z)-4n (R = cyclo-pentyl) | 22.5 | 35.0 | 0 | 13.7 | 19.8 | 0 | 23.9 | 51.0 |
| (Z)-4o (R = cyclo-hexyl) | 25.6 | 30.0 | 28.8 | 21.1 | 15.9 | 16.0 | 23.9 | 40.8 |
| (Z)-4p (R = α-furyl) | 60.0 | 64.5 | 77.7 | 53.8 | 36.5 | 54.6 | 43.3 | 54.4 |
| (Z)-4q (R = α-thienyl) | 48.9 | 37.3 | 87.4 | 41.3 | 33.5 | 42.1 | 33.8 | 48.9 |
| (Z)-4r (R = β-pyridyl) | 76.7 | 82.7 | 97.1 | 66.3 | 74.7 | 93.9 | 76.7 | 93.3 |
| (Z)-4s (R = α-Cl-β-pyridyl) | 15.6 | 37.3 | 55.2 | 41.3 | 54.1 | 63.6 | 24.3 | 21.1 |
| (Z)-4t (R = n-pentyl) | 21.1 | 19.1 | 58.4 | 35.0 | 48.2 | 67.1 | 24.3 | 21.1 |
| (Z)-4u (R = n-ethyl) | 15.6 | 19.1 | 58.4 | 28.8 | 48.2 | 36.8 | 29.0 | 21.1 |
| (Z)-4v (R = n-propyl) | 15.6 | 28.2 | 32.6 | 41.3 | 57.1 | 54.6 | 24.3 | 21.1 |
| (Z)-4w (R = n-butyl) | 15.6 | 19.1 | 51.9 | 22.5 | 18.8 | 18.9 | 19.5 | 21.1 |
| (E)-4f′ (R = p-FPh) | 60.0 | 64.5 | 87.4 | 53.8 | 65.9 | 76.1 | 76.7 | 54.4 |
| (Z)-3a | 15.6 | 23.6 | 51.9 | 35.0 | 18.8 | 24.3 | 24.3 | 26.7 |
| (E)-3b | 15.6 | 23.6 | 51.9 | 22.5 | 18.8 | 27.9 | 19.5 | 21.1 |
| 2 | 21.1 | 19.1 | 45.5 | 16.3 | 24.7 | 18.9 | 24.3 | 15.6 |
| Chlorothanil | 100 | 73.3 | 75.0 | 73.9 | 73.1 | 96.1 | 90.4 | 91.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Duan, W.-G.; Lin, G.-S.; Li, K.; Hu, Q. Synthesis and Antifungal Activity of Novel 3-Caren-5-One Oxime Esters. Molecules 2017, 22, 1538. https://doi.org/10.3390/molecules22091538
Huang M, Duan W-G, Lin G-S, Li K, Hu Q. Synthesis and Antifungal Activity of Novel 3-Caren-5-One Oxime Esters. Molecules. 2017; 22(9):1538. https://doi.org/10.3390/molecules22091538
Chicago/Turabian StyleHuang, Min, Wen-Gui Duan, Gui-Shan Lin, Kun Li, and Qiong Hu. 2017. "Synthesis and Antifungal Activity of Novel 3-Caren-5-One Oxime Esters" Molecules 22, no. 9: 1538. https://doi.org/10.3390/molecules22091538
APA StyleHuang, M., Duan, W. -G., Lin, G. -S., Li, K., & Hu, Q. (2017). Synthesis and Antifungal Activity of Novel 3-Caren-5-One Oxime Esters. Molecules, 22(9), 1538. https://doi.org/10.3390/molecules22091538