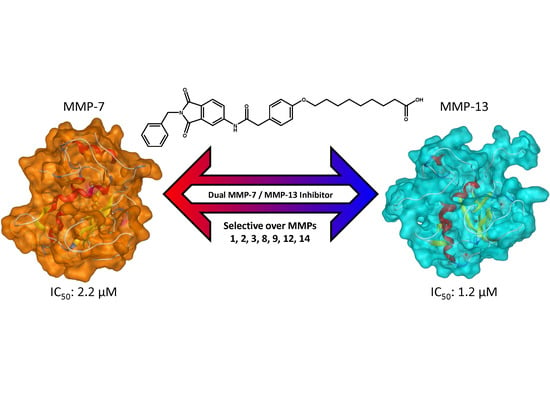
4.2. Chemistry
5-Amino-2-methyl-2,3-dihydro-1H-isoindole-1,3-dione (11a; ZHAWOC3444): Potassium hydroxide (0.35 g, 6.17 mmol) was added to a solution of 4-aminophthalimide 10 (1.00 g, 6.17 mmol) in dimethylformamide (30 mL) and the mixture was stirred at ambient temperature for 2 h. Iodomethane (0.88 g, 6.17 mmol) was added and it was stirred for another 18 h at the same temperature. Water (50 mL) and ethyl acetate (50 mL) was added and the resulting phases were separated. The organic phase was washed with brine, dried over sodium sulfate and concentrated in vacuum. Purification by chromatography on silica gel (Gradient: 0–100% ethyl acetate in cyclohexane) afforded the title compound 11a as a yellow solid (1.00 g, 92% yield): 1H-NMR (DMSO-d6): δ = 7.46 (d, J = 8.24 Hz, 1H), 6.90 (d, J = 2.00 Hz, 1H), 6.77 (dd, J = 8.24 Hz, 2.00 Hz, 1H), 6.42 (br. s, 2H), 2.94 (s, 3H) ppm. 13C-NMR (DMSO-d6): δ = 168.87, 168.57, 155.27, 135.10, 125.17, 117.33, 116.84, 107.42, 23.83 ppm. MS (m/z): 177 [M + H]+.
In analogy to ZHAWOC3444 the following derivatives were synthesized, employing the alkyl bromide instead of the alkyl iodide:
5-Amino-2-benzyl-2,3-dihydro-1H-isoindole-1,3-dione (11b; ZHAWOC899): The title compound 11b was obtained as a yellow solid in 59% yield: 1H-NMR DMSO-d6): δ = 7.51 (d, J = 8.04 Hz, 1H), 7.21–7.38 (m, 5H), 6.96 (d, J = 1.94 Hz, 1H), 6.82 (dd, J = 8.04 Hz, 1.94 Hz, 1H), 6.51 (s, 2H), 4.68 (s, 2H) ppm. 13C-NMR (DMSO-d6): δ = 168.06, 167.69, 155.07, 137.20, 134.41, 128.51, 127.25, 125.02, 116.67, 116.44, 107.09, 40.43 ppm. MS (m/z): 253 [M + H]+.
5-Amino-2-[(4-fluorophenyl)methyl]-2,3-dihydro-1H-isoindole-1,3-dione (11c; ZHAWOC3199): The title compound 11c was obtained as a yellow solid in 68% yield: 1H-NMR DMSO-d6): δ = 7.49 (d, J = 8.14 Hz, 1H), 7.33–7.28 (m, 2H), 7.17–7.11 (m, 2H), 6.93 (d, J = 1.94 Hz, 1H), 6.80 (dd, J = 8.14 Hz, 1.94 Hz, 1H), 6.50 (s, 2H), 4.65 (s, 2H) ppm. 13C-NMR (DMSO-d6): δ = 168.50, 168.11, 161.82 (d, J = 243.45 Hz, 1C), 155.55, 134.88, 133.90 (d, J = 3.02 Hz, 1C), 129.95 (d, J = 8.25 Hz, 2C), 125.52, 117.14, 116.87, 115.76 (d, J = 21.43 Hz, 2C), 107.58, 40.22 ppm. MS (m/z): 271 [M + H]+.
{[(7-Bromoheptyl)oxy]methyl}benzene (7a; ZHAWOC7096): 1-bromoheptanol (5.00 g, 25.62 mmol) was dissolved in tetrahydrofuran (45 mL) under an argon atmosphere. Benzylbromide (4.5 mL, 38.43 mmol) and sodium hydride (2.05 g, 85.4 mmol) were added and the mixture was stirred for 3 days. The reaction was quenched with saturated sodium hydrogen carbonate and diluted with water (20 mL) and extracted with diethyl ether (3 × 30 mL). The combined organic extracts were dried over sodium sulphate and concentrated in vacuum. Purification by chromatography on silica gel (Gradient: 0–100% ethyl acetate in cyclohexane) afforded the title compound 7a as a colorless oil (6.86 g, 94% yield): 1H-NMR (CDCl3): δ = 7.42–7.28 (m, 5H), 4.53 (s, 2H), 3.49 (t, J = 6.59 Hz, 2H), 3.43 (t, J = 6.82 Hz, 2H), 1.91–1.84 (m, 2H), 1.69–1.61 (m, 2H), 1.50–1.33 (m, 6H) ppm. 13C-NMR (CDCl3): δ = 138.67, 128.36, 127.63, 127.50, 72.90, 70.34, 33.98, 32.76, 29.67, 28.60, 28.12, 26.04 ppm. MS (m/z): 285 [M + H]+.
In analogy to ZHAWOC7096 the following derivatives were synthesized:
{[(8-Bromooctyl)oxy]methyl}benzene (7b; ZHAWOC6856): The title compound 7b was obtained as a colorless oil in 91% yield: 1H-NMR (DMSO-d6): δ = 7.37–7.25 (m, 5H), 4.44 (s, 2H), 3.51 (t, J = 6.73 Hz, 2H), 3.41 (t, J = 6.52 Hz, 2H), 1.81–1.74 (m, 2H), 1.56-1.49 (m, 2H), 1.39–1.23 (m, 8H) ppm. 13C-NMR (DMSO-d6): δ = 139.20, 128.66, 127.84, 127.75, 72.25, 70.03, 35.67, 32.69, 29.61, 29.13, 28.54, 27.94, 26.08 ppm. MS (m/z): 299 [M + H]+.
{[(9-Bromononyl)oxy]methyl}benzene (7c; ZHAWOC6852): The title compound 7c was obtained as a colorless oil in 90% yield: 1H-NMR (CDCl3): δ = 7.37–7.25 (m, 5H), 4.50 (s, 2H), 3.46 (t, J = 6.62 Hz, 2H), 3.40 (t, J = 6.89 Hz, 2H), 1.88–1.81 (m, 2H), 1.64–1.57 (m, 2H), 1.45–1.27 (m, 10H) ppm. 13C-NMR (CDCl3): δ = 138.73, 128.36, 127.64, 127.49, 72.89, 70.49, 34.04, 32.84, 29.77, 29.39, 29.37, 28.72, 28.17, 26.17 ppm. MS (m/z): 313 [M + H]+.
{[(10-Bromodecyl)oxy]methyl}benzene (7d; ZHAWOC6853): The title compound 7d was obtained as a colorless oil in 76% yield: 1H-NMR (CDCl3): δ = 7.37–7.24 (m, 5H), 4.50 (s, 2H), 3.46 (t, J = 6.62 Hz, 2H), 3.39 (t, J = 6.82 Hz, 2H), 1.87–1.81 (m, 2H), 1.64–1.57 (m, 2H), 1.44–1.26 (m, 12H) ppm. 13C-NMR (CDCl3): δ = 138.75, 128.36, 127.63, 127.48, 72.89, 70.52, 34.06, 32.86, 29.79, 29.49, 29.45, 29.39, 28.77, 28.19, 26.20 ppm. MS (m/z): 327 [M + H]+.
Methyl 2-{4-[2-(benzyloxy)ethoxy]phenyl}acetate (8a; ZHAWOC7100): Under an argon atmosphere, methyl 2-(4-hydroxyphenyl)acetate (3.51 g, 21.14 mmol) and caesium carbonate (13.78 g, 42.28 mmol) were suspended in dimethylformamide (130 mL), the mixture was stirred at ambient temperature for 2 h. Benzyl-2-bromoethylether (5.00 g, 23.25 mmol) was added and it was stirred at ambient temperature for further 12 h. Water (250 mL) and ethyl acetate (250 mL) were added and the resulting phases separated. The organic phase was dried over sodium sulfate and concentrated in vacuum. Purification by chromatography on silica gel (gradient: 0–100% ethyl acetate in cyclohexane) afforded the title compound 8a as a white solid (5.30 g, 84% yield): 1H-NMR (DMSO-d6): δ = 7.38–7.26 (m, 5H), 7.19–7.15 (m, 2H), 6.92–6.88 (m, 2H), 4.55 (s, 2H), 4.13–4.10 (m, 2H), 3.78–3.74 (m, 2H), 3.60 (s, 3H), 3.59 (s, 2H) ppm. 13C-NMR (DMSO-d6): δ = 172.33, 157.88, 138.78, 130.83, 128.70, 127.99, 127.89, 126.83, 114.83, 72.55, 68.71, 67.53, 52.06, 39.72 ppm. MS (m/z): 301 [M + H]+.
In analogy to ZHAWOC7100 the following derivatives were synthesized:
Methyl 2-{4-[3-(benzyloxy)propoxy]phenyl}acetate (8b; ZHAWOC4496): The title compound 8b was obtained as a white solid (1.46 g, 77% yield): 1H-NMR (CDCl3): δ = 7.35–7.20 (m, 5H), 7.19–7.13 (m, 2H), 6.87–6.79 (m, 2H), 4.49 (s, 2H), 4.04 (t, J = 6.24 Hz, 2H), 3.65 (s, 3H), 3.63 (t, J = 6.15 Hz, 2H), 3.53 (s, 2H), 2.05 (quint., J = 6.20 Hz, 2H) ppm. 13C-NMR (CDCl3): δ = 172.39, 158.21, 138.49, 130.30, 128.43, 127.65, 127.61, 1276.04, 114.69, 73.07, 66.86, 64.89, 51.99, 40.33, 29.80 ppm. MS (m/z): 337 [M + Na]+.
Methyl 2-{4-[4-(benzyloxy)butoxy]phenyl}acetate (8c; ZHAWOC4534): The title compound 8c was obtained as a white solid in 68% yield: 1H-NMR (CDCl3): δ = 7.37–7.22 (m, 5H), 7.20–7.12 (m, 2H), 6.86–6.79 (m, 2H), 4.50 (s, 2H), 3.95 (t, J = 6.08 Hz, 2H), 3.66 (s, 3H), 3.54 (s, 2H), 3.53 (t, J = 6.08 Hz, 2H), 1.93–1.72 (m, 4H) ppm. 13C-NMR (CDCl3): δ = 172.40, 158.22, 138.59, 130.26, 128.40, 127.66, 127.57, 125.93, 114.63, 72.94, 69.95, 67.67, 51.99, 40.32, 26.40, 26.18 ppm. MS (m/z): 351 [M + Na]+.
Methyl 2-(4-{[5-(benzyloxy)pentyl]oxy}phenyl)acetate (8d; ZHAWOC5921): The title compound 8d was obtained as a white solid in 90% yield: 1H-NMR (CDCl3): δ = 7.43–7.30 (m, 5H), 7.26–7.21 (m, 2H), 6.93–6.88 (m, 2H), 4.56 (s, 2H), 3.99 (t, J = 6.46 Hz, 2H), 3.72 (s, 3H), 3.61 (s, 2H), 3.56 (t, J = 6.46 Hz, 2H), 1.89–1.81 (m, 2H), 1.79–1.72 (m, 2H), 1.66–1.58 (m, 2H) ppm. 13C-NMR (CDCl3): δ = 172.22, 158.18, 138.59, 130.16, 128.28, 127.53, 127.42, 125.82, 114.51, 72.84, 70.15, 67.71, 51.82, 40.20, 29.48, 29.05, 22.77 ppm. MS (m/z): 343 [M + H]+.
Methyl 2-(4-{[6-(benzyloxy)hexyl]oxy}phenyl)acetate (8e; ZHAWOC5946): The title compound 8e was obtained as a white solid in 74% yield: 1H-NMR (CDCl3): δ = 7.39–7.34 (m, 4H), 7.32–7.27 (m, 1H), 7.22–7.18 (m, 2H), 6.88–6.84 (m, 2H), 4.53 (s, 2H), 3.95 (t, J = 6.51 Hz, 2H), 3.70 (s, 3H), 3.58 (s, 2H), 3.50 (t, J = 6.51 Hz, 2H), 1.84–1.76 (m, 2H), 1.71–1.64 (m, 2H), 1.53–1.43 (m, 4H) ppm. 13C-NMR (CDCl3): δ = 172.42, 158.31, 138.73, 130.29, 128.41, 127.68, 127.55, 125.91, 114.65, 72.94, 70.37, 67.92, 52.02, 40.37, 29.78, 29.30, 26.07, 25.99 ppm. MS (m/z): 357 [M + H]+.
Methyl 2-(4-{[7-(benzyloxy)heptyl]oxy}phenyl)acetate (8f; ZHAWOC7097): The title compound 8f was obtained as a white solid in 78% yield: 1H-NMR (CDCl3): δ = 7.37–7.29 (m, 4H), 7.29–7.22 (m, 1H), 7.18–7.13 (m, 2H), 6.86–6.79 (m, 2H), 4.49 (s, 2H), 3.91 (t, J = 6.63 Hz, 2H), 3.66 (s, 3H), 3.54 (s, 2H), 3.46 (t, J = 6.63 Hz, 2H), 1.79–1.71 (m, 2H), 1.66–1.59 (m, 2H), 1.49–1.31 (m, 6H) ppm. 13C-NMR (CDCl3): δ = 172.38, 158.30, 138.74, 130.7425, 128.37, 127.63, 127.50, 125.86, 114.62, 72.89, 70.44, 67.94, 51.99, 40.34, 29.75, 29.25, 26.19, 26.05 ppm. MS (m/z): 371 [M + H]+.
Methyl 2-(4-{[8-(benzyloxy)octyl]oxy}phenyl)acetate (8g; ZHAWOC6857): The title compound 8g was obtained as a white solid in 69% yield: 1H-NMR (DMSO-d6): δ = 7.36–7.24 (m, 5H), 7.18–7.13 (m, 2H), 6.88–6.83 (m, 2H), 4.43 (s, 2H), 3.92 (t, J = 6.31 Hz, 2H), 3.59 (s, 3H), 3.58 (s, 2H), 3.41 (t, J = 6.31 Hz, 2H), 1.71–1.64 (m, 2H), 1.56–1.50 (m, 2H), 1.42–1.24 (m, 8H) ppm. 13C-NMR (DMSO-d6): δ = 172.36, 158.06, 139.21, 130.79, 128.66, 127.84, 127.75, 126.54, 114.74, 72.25, 70.05, 67.81, 52.07, 39.71, 29.64, 29.25, 29.20, 29.13, 26.12, 25.94 ppm. MS (m/z): 385 [M + H]+.
Methyl 2-(4-{[9-(benzyloxy)nonyl]oxy}phenyl)acetate (8h; ZHAWOC6854): The title compound 8h was obtained as a white solid in 55% yield: 1H-NMR (CDCl3): δ = 7.36–7.31 (m, 4H), 7.29–24 (m, 1H), 7.20–7.14 (m, 2H), 6.86–6.82 (m, 2H), 4.50 (s, 2H), 3.92 (t, J = 6.83 Hz, 2H), 3.67 (s, 3H), 3.55 (s, 2H), 3.46 (t, J = 6.74 Hz, 2H), 1.79–1.72 (m, 2H), 1.64–1.58 (m, 2H), 1.46–1.27 (m, 10H) ppm. 13C-NMR (CDCl3): δ = 172.40, 158.30, 138.74, 130.23, 128.35, 127.62, 127.47, 125.82, 114.61, 72.88, 70.51, 68.00, 51.99, 40.33, 29.79, 29.53, 29.43, 29.35, 29.29, 26.21, 26.05 ppm. MS (m/z): 399 [M + H]+.
Methyl 2-(4-{[10-(benzyloxy)decyl]oxy}phenyl)acetate (8i; ZHAWOC6855): The title compound 8i was obtained as a white solid in 49% yield: 1H-NMR (CDCl3): δ = 7.35–7.31 (m, 4H), 7.29–24 (m, 1H), 7.19–7.14 (m, 2H), 6.86–6.82 (m, 2H), 4.49 (s, 2H), 3.92 (t, J = 6.65 Hz, 2H), 3.67 (s, 3H), 3.54 (s, 2H), 3.46 (t, J = 6.65 Hz, 2H), 1.79–1.72 (m, 2H), 1.64–1.58 (m, 2H), 1.46–1.27 (m, 12H) ppm. 13C-NMR (CDCl3): δ = 172.40, 158.33, 138.78, 130.25, 128.36, 127.63, 127.48, 125.84, 114.62, 72.89, 70.54, 68.01, 51.98, 40.33, 29.82, 29.56, 29.55, 29.50, 29.41, 29.31, 26.23, 26.08 ppm. MS (m/z): 413 [M + H]+.
2-{4-[2-(Benzyloxy)ethoxy]phenyl}acetic acid (9a; ZHAWOC7101): The ester (8a) (5.20 g, 11.33 mmol) was dissolved in methanol (300 mL) and stirred at ambient temperature. Potassium hydroxide 10% in water (300 mL) was added over 10 min. and the mixture was stirred for another 20 min. Methanol was removed in vacuum and the aqueous phase extracted with diethyl ether (200 mL). The aqueous phase was acidified with concentrated hydrochloric acid and extracted with diethyl ether (300 mL). The second organic phase was dried over sodium sulfate and concentrated in vacuum to obtain the title compound 9a as a white solid (4.71 g, 95% yield): 1H–NMR (DMSO-d6): δ = 12.25 (s, 1H), 7.38–7.33 (m, 4H), 7.31–7.26 (m, 1H), 7.20–7.14 (m, 2H), 6.92–6.86 (m, 2H), 4.55 (s, 2H), 4.13–4.09 (m, 2H), 3.78–3.74 (m, 2H), 3.49 (s, 2H) ppm. 13C-NMR (DMSO-d6): δ = 173.44, 157.72, 138.78, 130.84, 128.71, 128.00, 127.89, 127.53, 114.73, 72.56, 68.72, 67.53, 40.26 ppm. MS (m/z): 285 [M − H]−.
In analogy to ZHAWOC7101 the following derivatives were synthesized:
2-{4-[3-(Benzyloxy)propoxy]phenyl}acetic acid (9b; ZHAWOC4497): The title compound 9b was obtained as a white solid (0.48 g, 98% yield): 1H-NMR (DMSO-d6): δ = 12.24 (br. s, 1H), 7.36–7.23 (m, 5H), 7.20–7.11 (m, 2H), 6.90–6.80 (m, 2H), 4.48 (s, 2H), 4.02 (t, J = 6.33 Hz, 2H), 3.59 (t, J = 6.33 Hz, 2H), 3.49 (s, 2H), 1.99 (quint., J = 6.33 Hz, 4H) ppm. 13C-NMR (DMSO-d6: δ = 173.43, 157.83, 139.00, 130.79, 128.66, 127.83, 127.77, 127.38, 114.67, 72.40, 66.76, 64.99, 40.29, 29.64 ppm. MS (m/z): 299 [M − H]−.
2-{4-[4-(Benzyloxy)butoxy]phenyl}acetic acid (9c; ZHAWOC4535): The title compound 9c was obtained as a white solid in 90% yield: 1H-NMR (CDCl3): δ = 8.90 (br. s, 1H), 7.36–7.23 (m, 5H), 7.19–7.11 (m, 2H), 6.86–6.78 (m, 2H), 4.51 (s, 2H), 3.94 (t, J = 6.03 Hz, 2H), 3.55 (s, 2H), 3.53 (t, J = 6.03 Hz, 2H), 1.92–1.71 (m, 4H) ppm. 13C-NMR (CDCl3): δ = 177.93, 158.34, 138.47, 130.41, 128.42, 127.73, 127.62, 125.32, 114.68, 72.93, 69.93, 67.67, 40.20, 26.35, 26.14 ppm. MS (m/z): 313 [M − H]−.
2-(4-{[5-(Benzyloxy)pentyl]oxy}phenyl)acetic acid (9d; ZHAWOC5922): The title compound 9d was obtained as a white solid in 91% yield: 1H-NMR (CDCl3): δ = 7.36–7.26 (m, 5H), 7.20–7.16 (m, 2H), 6.88–6.83 (m, 2H), 4.53 (s, 2H), 3.94 (t, J = 6.48 Hz, 2H), 3.55 (s, 2H), 3.57 (t, J = 6.48 Hz, 2H), 1.83–1.76 (m, 2H), 1.74–1.67 (m, 2H), 1.59–1.51 (m, 2H) ppm. 13C-NMR (CDCl3): δ = 177.20, 158.31, 138.48, 130.39, 128.40, 127.71, 127.59, 125.48, 114.64, 72.90, 70.21, 67.83, 40.21, 29.47, 29.09, 22.79 ppm. MS (m/z): 327 [M − H]−.
2-(4-{[6-(Benzyloxy)hexyl]oxy}phenyl)acetic acid (9e; ZHAWOC5947): The title compound 9e was obtained as a white solid in 73% yield: 1H-NMR (DMSO-d6): δ = 12.23 (br. s, 1H), 7.36–7.23 (m, 5H), 7.18–7.12 (m, 2H), 6.87–6.81 (m, 2H), 4.44 (s, 2H), 3.91 (t, J = 6.50 Hz, 2H), 3.47 (s, 2H), 3.42 (t, J = 6.50 Hz, 2H), 1.73–1.65 (m, 2H), 1.59–1.52 (m, 2H), 1.45–1.33 (m, 4H) ppm. 13C-NMR (DMSO-d6): δ = 173.45, 157.90, 139.20, 130.79, 128.66, 127.84, 127.75, 127.25, 114.64, 72.27, 70.01, 67.77, 40.26, 29.63, 29.14, 25.96, 25.85 ppm. MS (m/z): 341 [M − H]−.
2-(4-{[7-(Benzyloxy)heptyl]oxy}phenyl)acetic acid (9f; ZHAWOC7098): The title compound 9f was obtained as a white solid in 94% yield: 1H-NMR (CDCl3): δ = 7.35–7.32 (m, 4H), 7.30–7.25 (m, 1H), 7.20–7.16 (m, 2H), 6.87–6.83 (m, 2H), 4.50 (s, 2H), 3.92 (t, J = 6.57 Hz, 2H), 3.58 (s, 2H), 3.47 (t, J = 6.66 Hz, 2H), 1.80–1.73 (m, 2H), 1.66–1.59 (m, 2H), 1.48–1.34 (m, 6H) ppm. 13C-NMR (CDCl3): δ = 158.43, 138.67, 130.34, 128.35, 127.63, 127.48, 125.12, 114.67, 72.87, 70.42, 67.95, 39.85, 29.20, 26.14, 26.00 ppm. MS (m/z): 355 [M − H]−.
2-(4-{[8-(Benzyloxy)octyl]oxy}phenyl)acetic acid (9g; ZHAWOC6858): The title compound 9g was obtained as a white solid in 96% yield: 1H–NMR (DMSO-d6): δ = 12.23 (br. s, 1H), 7.36–7.24 (m, 5H), 7.16–7.11 (m, 2H), 6.87–6.82 (m, 2H), 4.44 (s, 2H), 3.91 (t, J = 6.50 Hz, 2H), 3.47 (s, 2H), 3.41 (t, J = 6.55 Hz, 2H), 1.72–1.64 (m, 2H), 1.57–1.50 (m, 2H), 1.42–1.23 (m, 8H) ppm. 13C-NMR (DMSO-d6,): δ = 173.45, 157.89, 139.21, 130.78, 128.66, 127.84, 127.75, 127.27, 114.63, 72.24, 70.05, 67.81, 40.27, 29.64, 29.25, 29.20, 29.14, 26.12, 25.95 ppm. MS (m/z): 369 [M − H]−.
2-(4-{[9-(Benzyloxy)nonyl]oxy}phenyl)acetic acid (9h; ZHAWOC6861): The title compound 9h was obtained as a white solid in 81% yield: 1H–NMR (DMSO-d6): δ = 12.24 (br. s, 1H), 7.36–7.24 (m, 5H), 7.16–7.11 (m, 2H), 6.87–6.82 (m, 2H), 4.43 (s, 2H), 3.91 (t, J = 6.56 Hz, 2H), 3.46 (s, 2H), 3.40 (t, J = 6.56 Hz, 2H), 1.72–1.64 (m, 2H), 1.57–1.49 (m, 2H), 1.42–1.23 (m, 10H) ppm. 13C-NMR (DMSO-d6): δ = 173.45, 157.89, 139.21, 130.78, 128.66, 127.83, 127.74, 127.27, 114.63, 72.24, 70.05, 67.81, 40.27, 29.65, 29.43, 29.25, 29.20, 29.16, 26.15, 25.98 ppm. MS (m/z): 383 [M − H]−.
2-(4-{[10-(Benzyloxy)decyl]oxy}phenyl)acetic acid (9i; ZHAWOC6862): The title compound 9i was obtained as a white solid in 99% yield: 1H-NMR (DMSO-d6): δ = 12.24 (br. s, 1H), 7.36–7.24 (m, 5H), 7.16–7.11 (m, 2H), 6.87–6.82 (m, 2H), 4.43 (s, 2H), 3.91 (t, J = 6.38 Hz, 2H), 3.46 (s, 2H), 3.40 (t, J = 6.38 Hz, 2H), 1.72–1.64 (m, 2H), 1.57–1.49 (m, 2H), 1.42–1.23 (m, 12H) ppm. 13C-NMR (DMSO-d6): δ = 173.45, 157.89, 139.21, 130.79, 128.66, 127.83, 127.74, 127.27, 114.63, 72.24, 70.06, 67.81, 40.27, 29.65, 29.42, 29.28, 29.22, 29.16, 26.16, 25.99 ppm. MS (m/z): 397 [M − H]−.
2-(4-{[5-(Benzyloxy)pentyl]oxy}phenyl)-N-(2-methyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)acetamide (12a; ZHAWOC6647): The acid 9d (0.92 g, 2.80 mmol) was stirred in an excess of thionyl chloride at 55 °C for 1 h. After removal of excess thionyl chloride under vacuum, the acid chloride was dissolved in tetrahydrofuran (2 mL) and added to a solution of 5-amino-2-methylisoindoline-1,3-dione (11a, 0.41 g, 2.33 mmol) in tetrahydrofuran (12 mL) under argon at ambient temperature. Diisopropylethylamine (1.00 mL) was added and the mixture was stirred at ambient temperature for 2 h. After removal of the tetrahydrofuran in vacuum, ethyl acetate (70 mL) and 10% citric acid (70 mL) were added and the resulting phases were separated. The organic phase was washed with 10% sodium bicarbonate (70 mL) and brine (70 mL), dried over sodium sulfate and concentrated in vacuum. Purification by chromatography on silica gel (Gradient: 0–100% ethyl acetate in cyclohexane) afforded the title compound 12a as a yellow solid (0.12 g, 11% yield): 1H-NMR (DMSO-d6): δ = 10.69 (s, 1H), 8.18 (d, J = 1.83 Hz, 1H), 7.85 (dd, J = 8.19 Hz, 1.83 Hz, 1H), 7.79 (d, J = 8.19 Hz, 1H), 7.35–7.21 (m, 7H), 6.89–6.85 (m, 2H), 4.44 (s. 2H), 3.93 (t, J = 6.52 Hz, 2H), 3.63 (s, 2H), 3.43 (t, J = 6.47 Hz, 2H), 3.00 (s, 3H), 1.74–1.66 (m, 2H), 1.63–1.56 (m, 2H), 1.50–1.42 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.90, 168.26, 168.12, 158.03, 145.04, 139.18, 133.82, 130.64, 128.68, 127.86, 127.77, 127.55, 126.09, 124.55, 123.52, 114.82, 113.09, 72.28, 69.98, 67.80, 42.97, 29.39, 28.96, 24.19, 22.85 ppm. MS (m/z): 487 [M + H]+.
In analogy to ZHAWOC6647 the following derivatives were synthesized:
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-{4-[2-(benzyloxy)ethoxy]phenyl}acetamide (12b; ZHAWOC5467): The title compound 12b was obtained as a yellow solid in 80% yield: 1H-NMR (DMSO-d6): δ = 10.74 (s, 1H), 8.21 (d, J = 1.85 Hz, 1H), 7.89 (dd, J = 8.21 Hz, 1.85 Hz, 1H), 7.84 (d, J = 8.21 Hz, 1H), 7.36–7.23 (m, 12H), 6.93–6.89 (m, 2H), 4.73 (s, 2H), 4.55 (s, 2H), 4.13–4.10 (m, 2H), 3.77–3.74 (m, 2H), 3.64 (s, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.90, 167.94, 167.76, 157.84, 145.30, 138.77, 137.21, 130.67, 129.04, 128.70, 127.99, 127.88, 127.86, 127.83, 127.76, 124.93, 114.89, 113.33, 72.53, 68.70, 67.54, 42.95, 41.31 ppm. MS (m/z): 521 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-{4-[3-(benzyloxy)propoxy]phenyl}acetamide (12c; ZHAWOC4511): The title compound 12c was obtained as a yellow solid (2.50g, 62% yield): 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.80 Hz, 1H), 7.89 (dd, J = 8.31 Hz, 1.80 Hz, 1H), 7.83 (d, J = 8.31 Hz, 1H), 7.35–7.21 (m, 12H), 6.91–6.85 (m, 2H), 4.73 (s, 2H), 4.47 (s, 2H), 4.02 (t, J = 6.35 Hz, 2H), 3.64 (s, 2H), 3.57 (t, J = 6.35 Hz, 2H), 1.97 (quint., J = 3.65 Hz, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.92, 167.93, 167.76, 157.93, 145.30, 139.00, 137.21, 133.54, 130.66, 129.04, 128.67, 127.84, 127.83, 127.79, 127.63, 124.92, 123.81, 114.83, 113.33, 72.35, 66.72, 65.02, 42.96, 41.31, 29.61 ppm. MS (m/z): 535 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-{4-[4-(benzyloxy)butoxy]phenyl}acetamide (12d; ZHAWOC4752): The title compound 12d was obtained as a yellow solid in 64% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.83 Hz, 1H), 7.89 (dd, J = 8.23 Hz, 1.83 Hz, 1H), 7.84 (d, J = 8.23 Hz, 1H), 7.35–7.21 (m, 12H), 6.89–6.85 (m, 2H), 4.73 (s, 2H), 4.45 (s. 2H), 3.95 (t, J = 6.27 Hz, 2H), 3.63 (s, 2H), 3.48 (t, J = 6.27 Hz, 2H), 1.79–1.72 (m, 2H), 1.72–1.64 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.95, 167.96, 167.78, 157.99, 145.32, 139.14, 137.23, 133.55, 130.65, 129.06, 128.69, 127.89, 127.87, 127.84, 127.78, 127.56, 125.73, 124.94, 123.83, 114.84, 113.34, 72.28, 69.75, 67.66, 42.97, 41.32, 26.29, 26.10 ppm. MS (m/z): 549 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-(4-{[5-(benzyloxy)pentyl]oxy}phenyl)acetamide (12e; ZHAWOC5979): The title compound 12e was obtained as a white solid in 44% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.83 Hz, 1H), 7.89 (dd, J = 8.23 Hz, 1.83 Hz, 1H), 7.84 (d, J = 8.23 Hz, 1H), 7.35–7.21 (m, 12H), 6.89–6.85 (m, 2H), 4.73 (s, 2H), 4.44 (s. 2H), 3.93 (t, J = 6.47 Hz, 2H), 3.63 (s, 2H), 3.43 (t, J = 6.39 Hz, 2H), 1.74–1.66 (m, 2H), 1.63–1.56 (m, 2H), 1.50–1.42 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.94, 167.76, 158.01, 145.30, 139.17, 137.21, 133.54, 130.63, 129.05, 128.67, 127.85, 127.83, 127.76, 127.51, 125.72, 124.93, 123.81, 114.82, 113.33, 72.27, 69.97, 67.79, 42.96, 41.31, 29.38, 28.95, 22.84 ppm. MS (m/z): 563 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-(4-{[6-(benzyloxy)hexyl]oxy}phenyl)acetamide (12f; ZHAWOC5980): The title compound 12f was obtained as a white solid in 34% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.83 Hz, 1H), 7.89 (dd, J = 8.23 Hz, 1.83 Hz, 1H), 7.83 (d, J = 8.23 Hz, 1H), 7.34–7.21 (m, 12H), 6.89–6.85 (m, 2H), 4.73 (s, 2H), 4.43 (s. 2H), 3.91 (t, J = 6.43 Hz, 2H), 3.63 (s, 2H), 3.41 (t, J = 6.43 Hz, 2H), 1.72–1.64 (m, 2H), 1.58–1.51 (m, 2H), 1.44–1.32 (m, 4H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.93, 167.75, 158.03, 145.31, 139.18, 137.21, 133.53, 130.63, 129.04, 128.65, 127.85, 127.84, 127.74, 127.50, 125.71, 124.91, 123.80, 114.80, 113.33, 72.25, 69.99, 67.79, 42.97, 41.31, 29.62, 29.11, 25.95, 25.83 ppm. MS (m/z): 577 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-(4-{[7-(benzyloxy)heptyl]oxy}phenyl)acetamide (12g; ZHAWOC7099): The title compound 12g was obtained as a white solid in 18% yield: 1H-NMR (DMSO-d6): δ = 10.72 (s, 1H), 8.21 (d, J = 1.80 Hz, 1H), 7.89 (dd, J = 8.24 Hz, 1.80 Hz, 1H), 7.84 (d, J = 8.24 Hz, 1H), 7.36–7.21 (m, 12H), 6.89–6.85 (m, 2H), 4.73 (s, 2H), 4.43 (s. 2H), 3.92 (t, J = 6.41 Hz, 2H), 3.63 (s, 2H), 3.41 (t, J = 6.41 Hz, 2H), 1.72–1.64 (m, 2H), 1.58–1.50 (m, 2H), 1.43–1.26 (m, 6H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.94, 167.76, 158.03, 145.31, 139.20, 137.21, 133.54, 130.63, 129.04, 128.66, 127.86, 127.83, 127.74, 127.50, 125.72, 124.93, 123.81, 114.81, 113.32, 72.24, 70.02, 67.80, 42.96, 41.31, 29.59, 29.09, 29.01, 26.12, 25.95 ppm. MS (m/z): 591 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-(4-{[8-(benzyloxy)octyl]oxy}phenyl)acetamide (12h; ZHAWOC7095): The title compound 12h was obtained as a white solid in 38% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.80 Hz, 1H), 7.89 (dd, J = 8.24 Hz, 1.80 Hz, 1H), 7.84 (d, J = 8.24 Hz, 1H), 7.35–7.21 (m, 12H), 6.89–6.85 (m, 2H), 4.74 (s, 2H), 4.43 (s. 2H), 3.92 (t, J = 6.47 Hz, 2H), 3.63 (s, 2H), 3.40 (t, J = 6.47 Hz, 2H), 1.72–1.64 (m, 2H), 1.56–1.49 (m, 2H), 1.42–1.24 (m, 8H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.94, 167.76, 158.03, 145.31, 139.20, 137.21, 133.54, 130.63, 129.04, 128.65, 127.86, 127.83, 127.74, 127.49, 125.71, 124.92, 123.81, 114.80, 113.32, 72.24, 70.04, 67.82, 42.96, 41.31, 29.63, 29.24, 29.19, 29.12, 26.11, 25.94 ppm. MS (m/z): 605 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-(4-{[9-(benzyloxy)nonyl]oxy}phenyl)acetamide (12i; ZHAWOC6931): The title compound 12i was obtained as a white solid in 63% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.80 Hz, 1H), 7.89 (dd, J = 8.25 Hz, 1.80 Hz, 1H), 7.83 (d, J = 8.25 Hz, 1H), 7.35–7.21 (m, 12H), 6.89–6.85 (m, 2H), 4.74 (s, 2H), 4.42 (s. 2H), 3.92 (t, J = 6.64 Hz, 2H), 3.63 (s, 2H), 3.40 (t, J = 6.64 Hz, 2H), 1.72–1.64 (m, 2H), 1.56–1.48 (m, 2H), 1.42–1.21 (m, 10H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.93, 167.76, 158.03, 145.31, 139.20, 137.21, 133.54, 130.63, 129.04, 128.65, 127.86, 127.83, 127.74, 127.49, 125.72, 124.92, 123.81, 114.80, 113.33, 72.24, 70.05, 67.83, 42.96, 41.31, 29.64, 29.42, 29.23, 29.17, 29.14, 26.14, 25.96 ppm. MS (m/z): 619 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-(4-{[10-(benzyloxy)decyl]oxy}phenyl)acetamide (12j; ZHAWOC6932): The title compound 12j was obtained as a white solid in 46% yield: 1H-NMR (DMSO-d6): δ = 10.74 (s, 1H), 8.21 (d, J = 1.85 Hz, 1H), 7.89 (dd, J = 8.23 Hz, 1.85 Hz, 1H), 7.84 (d, J = 8.23 Hz, 1H), 7.35–7.21 (m, 12H), 6.89–6.85 (m, 2H), 4.73 (s, 2H), 4.42 (s. 2H), 3.92 (t, J = 6.65 Hz, 2H), 3.63 (s, 2H), 3.40 (t, J = 6.65 Hz, 2H), 1.72–1.64 (m, 2H), 1.55–1.48 (m, 2H), 1.42–1.21 (m, 12H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.94, 167.76, 158.03, 145.31, 139.20, 137.21, 133.54, 130.63, 129.04, 128.65, 127.86, 127.83, 127.74, 127.49, 125.71, 124.92, 123.81, 114.80, 113.32, 72.23, 70.04, 67.83, 42.96, 41.31, 29.64, 29.40, 29.26, 29.19, 29.14, 26.15, 25.97 ppm. MS (m/z): 633 [M + H]+.
2-{4-[3-(Benzyloxy)propoxy]phenyl}-N-{2-[(4-fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}acetamide (12k; ZHAWOC6641): The title compound 12k was obtained as a yellow solid in 72% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.80 Hz, 1H), 7.89 (dd, J = 8.27 Hz, 1.80 Hz, 1H), 7.83 (d, J = 8.27 Hz, 1H), 7.36–7.28 (m, 6H), 7.26–7.22 (m, 3H), 7.17–7.11 (m, 2H), 6.90–6.85 (m, 2H), 4.71 (s, 2H), 4.47 (s, 2H), 4.02 (t, J = 6.50 Hz, 2H), 3.64 (s, 2H), 3.57 (t, J = 6.23 Hz, 2H), 2.00–1.94 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.94, 167.91, 167.73, 161.92 (d, J = 243.23 Hz, 1C), 157.95, 145.31, 139.01, 133.55, 133.45 (d, J = 3.05 Hz, 1C), 130.67, 130.09 (d, J = 7.75 Hz, 2C), 128.69, 127.86, 127.80, 127.64, 125.72, 124.93, 123.82, 115.82 (d, J = 21.40 Hz, 2C), 114.85, 113.34, 72.36, 66.73, 65.02, 42.96, 40.63, 29.61 ppm. MS (m/z): 553 [M + H]+.
2-{4-[4-(Benzyloxy)butoxy]phenyl}-N-{2-[(4-fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}acetamide (12l; ZHAWOC7102): The title compound 12l was obtained as a yellow solid in 55% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.89 Hz, 1H), 7.89 (dd, J = 8.21 Hz, 1.89 Hz, 1H), 7.83 (d, J = 8.21 Hz, 1H), 7.36–7.28 (m, 7H), 7.26–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.85 (m, 2H), 4.72 (s, 2H), 4.45 (s, 2H), 3.95 (t, J = 6.10 Hz, 2H), 3.63 (s, 2H), 3.48 (t, J = 6.20 Hz, 2H), 1.80–1.73 (m, 2H), 1.71–1.64 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.94, 167.92, 167.74, 161.92 (d, J = 243.56 Hz, 1C), 157.99, 145.32, 139.14, 133.56, 133.45 (d, J = 2.99 Hz, 1C), 130.65, 130.09 (d, J = 8.27 Hz, 2C), 128.69, 127.89, 127.78, 127.55, 125.73, 124.94, 123.81, 115.83 (d, J = 21.63 Hz, 2C), 114.83, 113.34, 72.28, 69.75, 67.66, 42.96, 40.63, 26.30, 26.10 ppm. MS (m/z): 567 [M + H]+.
2-(4-{[5-(Benzyloxy)pentyl]oxy}phenyl)-N-{2-[(4-fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-iso-indol-5-yl}acetamide (12m; ZHAWOC5682): The title compound 12m was obtained as a yellow solid in 58% yield: 1H-NMR (DMSO-d6): δ = 10.72 (s, 1H), 8.20 (d, J = 1.82 Hz, 1H), 7.88 (dd, J = 8.27 Hz, 1.82 Hz, 1H), 7.83 (d, J = 8.27 Hz, 1H), 7.36–7.21 (m, 9H), 7.17–7.11 (m, 2H), 6.90–6.85 (m, 2H), 4.72 (s, 2H), 4.44 (s, 2H), 3.92 (t, J = 6.45 Hz, 2H), 3.63 (s, 2H), 3.43 (t, J = 6.40 Hz, 2H), 1.73–1.66 (m, 2H), 1.62–1.55 (m, 2H), 1.50–1.42 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.92, 167.89, 167.71, 161.90 (d, J = 243.23 Hz, 1C), 158.01, 145.30, 139.17, 133.54, 133.45 (d, J = 3.06 Hz, 1C), 130.63, 130.08 (d, J = 8.28 Hz, 2C), 128.66, 127.84, 127.75, 127.50, 125.71, 124.92, 123.80, 115.81 (d, J = 21.43 Hz, 2C), 114.81, 113.33, 72.27, 69.97, 67.79, 42.96, 40.62, 29.38, 28.95, 22.84 ppm. MS (m/z): 603 [M + Na]+.
2-(4-{[6-(Benzyloxy)hexyl]oxy}phenyl)-N-{2-[(4-fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-iso-indol-5-yl}acetamide (12n; ZHAWOC6640): The title compound 12n was obtained as a yellow solid in 36% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.83 Hz, 1H), 7.89 (dd, J = 8.24 Hz, 1.83 Hz, 1H), 7.83 (d, J = 8.24 Hz, 1H), 7.36–7.26 (m, 7H), 7.25–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.89–6.85 (m, 2H), 4.72 (s, 2H), 4.43 (s, 2H), 3.92 (t, J = 6.38 Hz, 2H), 3.63 (s, 2H), 3.41 (t, J = 6.45 Hz, 2H), 1.73–1.65 (m, 2H), 1.59–1.51 (m, 2H), 1.44–1.33 (m, 4H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.89, 167.72, 161.90 (d, J = 243.56 Hz, 1C), 158.03, 145.31, 139.19, 133.54, 133.44 (d, J = 3.08 Hz, 1C), 130.63, 130.08 (d, J = 8.34 Hz, 2C), 128.66, 127.84, 127.74, 127.49, 125.71, 124.92, 123.80, 115.81 (d, J = 21.43 Hz, 2C), 114.80, 113.32, 72.25, 69.99, 67.79, 42.96, 40.62, 29.62, 29.11, 25.95, 25.83 ppm. MS (m/z): 595 [M + H]+.
2-(4-{[7-(Benzyloxy)heptyl]oxy}phenyl)-N-{2-[(4-fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-iso-indol-5-yl}acetamide (12o; ZHAWOC6635): The title compound 12o was obtained as a yellow solid in 47% yield: 1H-NMR (DMSO-d6): δ = 10.72 (s, 1H), 8.20 (d, J = 1.81 Hz, 1H), 7.88 (dd, J = 8.26 Hz, 1.81 Hz, 1H), 7.83 (d, J = 8.24 Hz, 1H), 7.36–7.26 (m, 7H), 7.25–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.89–6.85 (m, 2H), 4.71 (s, 2H), 4.42 (s, 2H), 3.91 (t, J = 6.35 Hz, 2H), 3.63 (s, 2H), 3.40 (t, J = 6.45 Hz, 2H), 1.71–1.64 (m, 2H), 1.57–1.49 (m, 2H), 1.42–1.27 (m, 6H) ppm. 13C-NMR (DMSO-d6): δ = 170.92, 167.89, 167.71, 161.90 (d, J = 242.72 Hz, 1C), 158.03, 145.30, 139.20, 133.54, 133.44 (d, J = 2.97 Hz, 1C), 130.63, 130.08 (d, J = 8.38 Hz, 2C), 128.65, 127.83, 127.73, 127.49, 125.71, 124.92, 123.80, 115.81 (d, J = 21.54 Hz, 2C), 114.80, 113.32, 72.24, 70.02, 67.80, 42.96, 40.62, 29.59, 29.09, 29.01, 26.12, 25.95 ppm. MS (m/z): 609 [M + H]+.
2-(4-{[8-(Benzyloxy)octyl]oxy}phenyl)-N-{2-[(4-fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-iso-indol-5-yl}acetamide (12p; ZHAWOC6638): The title compound 12p was obtained as a yellow solid in 57% yield: 1H-NMR (DMSO-d6): δ = 10.72 (s, 1H), 8.20 (d, J = 1.81 Hz, 1H), 7.89 (dd, J = 8.23 Hz, 1.81 Hz, 1H), 7.83 (d, J = 8.23 Hz, 1H), 7.37–7.26 (m, 7H), 7.25–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.89–6.85 (m, 2H), 4.71 (s, 2H), 4.42 (s, 2H), 3.91 (t, J = 6.51 Hz, 2H), 3.63 (s, 2H), 3.40 (t, J = 6.51 Hz, 2H), 1.71–1.64 (m, 2H), 1.57–1.49 (m, 2H), 1.42–1.25 (m, 8H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.90, 167.72, 161.90 (d, J = 243.01 Hz, 1C), 158.03, 145.30, 139.20, 133.55, 133.44 (d, J = 3.21 Hz, 1C), 130.63, 130.08 (d, J = 8.28 Hz, 2C), 128.65, 127.83, 127.74, 127.48, 125.71, 124.93, 123.80, 115.81 (d, J = 21.37 Hz, 2C), 114.80, 113.32, 72.24, 70.04, 67.82, 42.96, 40.62, 29.63, 29.24, 29.18, 29.12, 26.11, 25.93 ppm. MS (m/z): 623 [M + H]+.
2-{4-[(5-Hydroxypentyl)oxy]phenyl}-N-(2-methyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)acetamide (13a; ZHAWOC6648): The benzyl ether 12a (0.12 g, 0.25 mmol) was dissolved in dichloromethane (3.00 mL) under an argon atmosphere. Trimethylsilyl iodide (0.35 ml) was added and stirred at room temperature for 2 h. The reaction was quenched by an addition of methanol (10 mL). The solvent was removed in vacuum and the residue purified by column chromatography on silica gel (gradient: 0–100% methanol in dichloromethane) to afford the title compound 13a as a white solid (0.03 g, 28% yield): 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.18 (d, J = 1.86 Hz, 1H), 7.85 (dd, J = 8.22 Hz, 1.86 Hz, 1H), 7.80 (d, J = 8.22 Hz, 1H), 7.26–7.21 (m, 2H), 6.90–6.86 (m, 2H), 4.36 (t, J = 5.10 Hz, 1H), 3.93 (t, J = 6.45 Hz, 2H), 3.63 (s, 2H), 3.40 (td, J = 5.82 Hz, 5.10 Hz, 2H), 3.00 (s, 3H), 1.74–1.66 (m, 2H), 1.51–1.38 (m, 4H) ppm. 13C-NMR (DMSO-d6): δ = 170.92, 168.27, 168.13, 158.05, 145.06, 133.82, 130.65, 127.55, 126.08, 124.56, 123.5, 114.81, 113.10, 67.89, 61.08, 42.96, 32.69, 29.06, 24.19, 22.61 ppm. MS (m/z): 397 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-[4-(2-hydroxyethoxy)phenyl]acetamide (13b; ZHAWOC5473): The benzyl ether (12b) (0.77 g, 1.48 mmol) was dissolved in ethanol (70 mL). Palladium 10% on activated charcoal (0.15 g, 0.13 mmol) was added and a hydrogen atmosphere was applied at 1 bar. After stirring at ambient temperature for 2 h the mixture was filtered over celite and concentrated in vacuum. Purification by chromatography on silica gel (gradient: 0–100% ethyl acetate in cyclohexane) afforded the title compound 13b as a white solid (0.25 g, 39% yield): 1H-NMR (DMSO-d6): δ = 10.74 (br. s, 1H), 8.21 (d, J = 1.78 Hz, 1H), 7.89 (dd, J = 8.20 Hz, 1.78 Hz, 1H), 7.83 (d, J = 8.20 Hz, 1H), 7.34–7.22 (m, 7H), 6.92–6.87 (m, 2H), 4.84 (t, J = 4.79 Hz, 1H), 4.73 (s, 2H), 3.95 (t, J = 5.04 Hz, 2H), 3.70 (q, J = 4.45 Hz, 2H), 3.64 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.94, 167.76, 158.04, 145.32, 137.21, 133.54, 130.65, 129.04, 127.86, 127.83, 127.59, 125.71, 124.92, 123.81, 114.84, 113.33, 69.94, 60.04, 42.95, 41.31 ppm. MS (m/z): 431 [M + H]+.
In analogy to ZHAWOC5473 the following derivatives were synthesized:
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-[4-(3-hydroxypropoxy)phenyl]acetamide (13c; ZHAWOC4512): The title compound 13c was obtained as a white solid (0.65 g, 71% yield): 1H-NMR (DMSO-d6): δ = 10.74 (s, 1H), 8.21 (d, J = 1.81 Hz, 1H), 7.89 (dd, J = 8.25 Hz, 1.81 Hz, 1H), 7.84 (d, J = 8.25 Hz, 1H), 7.35–7.21 (m, 7H), 6.91–6.86 (m, 2H), 4.73 (s, 2H), 4.52 (t, J = 4.60 Hz, 1H), 4.00 (t, J = 6.42 Hz, 2H), 3.64 (s, 2H), 3.54 (m, 2H), 1.84 (quint., J = 6.31 Hz, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.94, 167.76, 158.03, 145.30, 137.21, 133.54, 130.64, 129.04, 127.86, 127.83, 127.51, 125.72, 124.92, 123.81, 114.80, 113.32, 64.99, 57.77, 42.95, 41.31, 32.60 ppm. MS (m/z): 445 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-[4-(4-hydroxybutoxy)phenyl]acetamide (13d; ZHAWOC4753): The title compound 13d was obtained as a white solid in 60% yield: 1H-NMR (DMSO-d6): δ = 10.74 (s, 1H), 8.21 (d, J = 1.83 Hz, 1H), 7.90 (dd, J = 8.24 Hz, 1.83 Hz, 1H), 7.84 (d, J = 8.24 Hz, 1H), 7.35–7.22 (m, 7H), 6.91–6.86 (m, 2H), 4.74 (s, 2H), 4.44 (t, J = 4.54 Hz, 1H), 4.95 (t, J = 6.44 Hz, 2H), 3.64 (s, 2H), 3.44 (m, 2H), 1.76–1.69 (m, 2H), 1.59–1.51 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.94, 167.94, 167.76, 158.02, 145.32, 137.21, 133.54, 130.54, 129.04, 127.86, 127.83, 127.50, 125.71, 124.92, 123.81, 114.81, 113.33, 67.82, 60.86, 42.96, 41.31, 29.47, 25.91 ppm. MS (m/z): 459 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-{4-[(5-hydroxypentyl)oxy]phenyl}acetamide (13e; ZHAWOC5130): The title compound 13e was obtained as a white solid in 24% yield: 1H-NMR (DMSO-d6): δ = 10.72 (s, 1H), 8.21 (d, J = 1.84 Hz, 1H), 7.89 (dd, J = 8.26 Hz, 1.84 Hz, 1H), 7.84 (d, J = 8.26 Hz, 1H), 7.35–7.25 (m, 5H), 7.25–7.21 (m, 2H), 6.90–6.85 (m, 2H), 4.73 (s, 2H), 4.36 (t, J = 5.16 Hz, 1H), 3.93 (t, J = 6.50 Hz, 2H), 3.63 (s, 2H), 3.40 (td, J = 6.20 Hz, 5.16 Hz, 2H), 1.73–1.66 (m, 2H), 1.50–1.38 (m, 4H) ppm. 13C-NMR (DMSO-d6): δ = 170.94, 167.94, 167.77, 158.04, 145.30, 137.21, 133.54, 130.64, 129.05, 127.86, 127.83, 127.49, 125.72, 124.93, 123.81, 114.81, 113.33, 67.89, 61.07, 42.96, 41.32, 32.68, 29.06, 22.61 ppm. MS (m/z): 473 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-{4-[(6-hydroxyhexyl)oxy]phenyl}acetamide (13f; ZHAWOC5132): The title compound 13f was obtained as a white solid in 6% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.82 Hz, 1H), 7.89 (dd, J = 8.23 Hz, 1.82 Hz, 1H), 7.84 (d, J = 8.23 Hz, 1H), 7.35–7.25 (m, 5H), 7.25–7.21 (m, 2H), 6.90–6.85 (m, 2H), 4.74 (s, 2H), 4.33 (t, J = 5.16 Hz, 1H), 3.92 (t, J = 6.50 Hz, 2H), 3.63 (s, 2H), 3.38 (td, J = 6.32 Hz, 5.16 Hz, 2H), 1.72–1.65 (m, 2H), 1.47–1.29 (m, 6H) ppm. 13C-NMR (DMSO-d6): δ = 170.94, 167.94, 167.77, 158.03, 145.30, 137.21, 133.54, 130.64, 129.05, 127.86, 127.83, 127.49, 125.72, 124.93, 123.81, 114.81, 113.33, 67.82, 61.10, 42.95, 41.32, 32.95, 29.22, 25.90, 25.74 ppm. MS (m/z): 487 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-{4-[(7-hydroxyheptyl)oxy]phenyl}acetamide (13g; ZHAWOC7103): The title compound 13g was obtained as a white solid in 24% yield: 1H-NMR (DMSO-d6): δ = 10.74 (s, 1H), 8.21 (d, J = 1.83 Hz, 1H), 7.89 (dd, J = 8.25 Hz, 1.83 Hz, 1H), 7.84 (d, J = 8.25 Hz, 1H), 7.35–7.21 (m, 7H), 6.90–6.86 (m, 2H), 4.74 (s, 2H), 4.32 (t, J = 4.98 Hz, 1H), 3.92 (t, J = 6.43 Hz, 2H), 3.63 (s, 2H), 3.37 (td, J = 6.43 Hz, 4.98 Hz, 2H), 1.72–1.65 (m, 2H), 1.45–1.25 (m, 8H) ppm. 13C-NMR (DMSO-d6): δ = 170.94, 167.94, 167.77, 158.03, 145.30, 137.21, 133.54, 130.64, 129.05, 127.86, 127.82, 127.49, 125.72, 124.93, 123.81, 114.81, 113.32, 67.83, 61.14, 42.96, 41.31, 32.94, 29.14, 29.12, 26.04, 25.93 ppm. MS (m/z): 501 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-{4-[(8-hydroxyoctyl)oxy]phenyl}acetamide (13h; ZHAWOC7137): The title compound 13h was obtained as a white solid in 59% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.80 Hz, 1H), 7.89 (dd, J = 8.20 Hz, 1.80 Hz, 1H), 7.84 (d, J = 8.20 Hz, 1H), 7.36–7.18 (m, 7H), 6.92–6.83 (m, 2H), 4.74 (s, 2H), 4.31 (t, J = 4.90 Hz, 1H), 3.92 (t, J = 6.13 Hz, 2H), 3.63 (s, 2H), 3.37 (td, J = 6.43 Hz, 4.90 Hz, 2H), 1.75–1.64 (m, 2H), 1.46–1.19 (m, 10H) ppm. 13C-NMR (DMSO-d6): δ = 170.95, 167.96, 167.78, 158.05, 145.32, 137.23, 133.56, 130.65, 129.06, 127.87, 127.84, 127.50, 125.73, 124.94, 123.82, 114.81, 113.33, 67.85, 61.18, 42.96, 41.32, 33.00, 29.38, 29.30, 29.16, 25.98, 25.93 ppm. MS (m/z): 515 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-{4-[(9-hydroxynonyl)oxy]phenyl}acetamide (13i; ZHAWOC6936): The title compound 13i was obtained as a white solid in 30% yield: 1H-NMR (DMSO-d6): δ = 10.74 (s, 1H), 8.21 (d, J = 1.83 Hz, 1H), 7.89 (dd, J = 8.24 Hz, 1.83 Hz, 1H), 7.83 (d, J = 8.24 Hz, 1H), 7.35–7.21 (m, 7H), 6.90–6.85 (m, 2H), 4.73 (s, 2H), 4.31 (t, J = 5.12 Hz, 1H), 3.92 (t, J = 6.46 Hz, 2H), 3.63 (s, 2H), 3.36 (td, J = 6.25 Hz, 5.12 Hz, 2H), 1.71–1.64 (m, 2H), 1.43–1.23 (m, 12H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.93, 167.76, 158.03, 145.31, 137.21, 133.54, 130.63, 129.04, 127.85, 127.83, 127.49, 125.71, 124.92, 123.81, 114.80, 113.33, 67.84, 61.18, 42.96, 41.31, 33.01, 29.53, 29.37, 29.22, 29.16, 26.00, 25.96 ppm. MS (m/z): 529 [M + H]+.
N-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-{4-[(10-hydroxydecyl)oxy]phenyl}acetamide (13j; ZHAWOC6937): The title compound 13j was obtained as a white solid in 18% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.21 (d, J = 1.80 Hz, 1H), 7.89 (dd, J = 8.16 Hz, 1.80 Hz, 1H), 7.84 (d, J = 8.16 Hz, 1H), 7.34–7.21 (m, 7H), 6.89–6.85 (m, 2H), 4.73 (s, 2H), 4.31 (t, J = 4.99 Hz, 1H), 3.92 (t, J = 6.46 Hz, 2H), 3.63 (s, 2H), 3.36 (td, J = 6.25 Hz, 4.99 Hz, 2H), 1.72–1.64 (m, 2H), 1.42–1.22 (m, 14H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.94, 167.76, 158.03, 145.30, 137.21, 133.54, 130.64, 129.04, 127.86, 127.83, 127.49, 125.72, 124.93, 123.80, 114.80, 113.32, 67.83, 61.18, 42.95, 41.31, 33.01, 29.51, 29.45 29.40, 29.24, 29.15, 26.81, 25.97 ppm. MS (m/z): 543 [M + H]+.
N-{2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}-2-[4-(3-hydroxypropoxy)-phenyl]acetamide (13k; ZHAWOC6642): The title compound 13k was obtained as a white solid in 34% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.20 (d, J = 1.99 Hz, 1H), 7.89 (dd, J = 8.24 Hz, 1.99 Hz, 1H), 7.83 (d, J = 8.24 Hz, 1H), 7.37–7.32 (m, 2H), 7.25–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.86 (m, 2H), 4.72 (s, 2H), 4.52 (t, J = 5.15 Hz, 1H), 4.00 (t, J = 6.53 Hz, 2H), 3.63 (s, 2H), 3.56–3.52 (m, 2H), 1.84 (quint. J = 6.25 Hz, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.95, 167.92, 167.74, 161.92 (d, J = 242.66 Hz, 1C), 158.05, 145.32, 133.56, 133.46 (d, J = 2.98 Hz, 1C), 130.65, 130.09 (d, J = 8.37 Hz, 2C), 127.52, 125.73, 124.94, 123.82, 115.83 (d, J = 21.42 Hz, 2C), 114.81, 113.34, 65.00, 57.78, 42.95, 40.63, 32.60 ppm. MS (m/z): 463 [M + H]+.
N-{2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}-2-[4-(4-hydroxybutoxy)-phenyl]acetamide (13l; ZHAWOC5462): The title compound 13l was obtained as a white solid in 15% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.20 (d, J = 1.83 Hz, 1H), 7.89 (dd, J = 8.22 Hz, 1.83 Hz, 1H), 7.83 (d, J = 8.22 Hz, 1H), 7.37–7.32 (m, 2H), 7.25–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.86 (m, 2H), 4.72 (s, 2H), 4.43 (t, J = 5.11 Hz, 1H), 3.94 (t, J = 6.58 Hz, 2H), 3.63 (s, 2H), 3.44 (td, J = 6.38 Hz, 5.11 Hz, 2H), 1.76–1.68 (m, 2H), 1.58–1.51 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.93, 167.90, 167.72, 161.90 (d, J = 243.27 Hz, 1C), 158.02, 145.30, 133.55, 133.44 (d, J = 3.06 Hz, 1C), 130.64, 130.08 (d, J = 8.27 Hz, 2C), 127.49, 125.71, 124.93, 123.80, 115.82 (d, J = 21.38 Hz, 2C), 114.81, 113.32, 67.82, 60.86, 42.95, 40.62, 29.47, 25.91 ppm. MS (m/z): 477 [M + H]+.
N-{2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}-2-{4-[(5-hydroxypentyl)oxy]-phenyl}acetamide (13m; ZHAWOC5683): The title compound 13m was obtained as a white solid in 44% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.20 (d, J = 1.84 Hz, 1H), 7.89 (dd, J = 8.20 Hz, 1.84 Hz, 1H), 7.83 (d, J = 8.20 Hz, 1H), 7.37–7.32 (m, 2H), 7.25–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.86 (m, 2H), 4.72 (s, 2H), 4.36 (t, J = 5.10 Hz, 1H), 3.93 (t, J = 6.45 Hz, 2H), 3.63 (s, 2H), 3.42–3.38 (m, 2H), 1.73–1.65 (m, 2H), 1.50–1.37 (m, 4H) ppm. 13C-NMR (DMSO-d6): δ = 170.94, 167.90, 167.72, 161.90 (d, J = 242.58 Hz, 1C), 158.04, 145.30, 133.55, 133.44 (d, J = 3.08 Hz, 1C), 130.64, 130.07 (d, J = 8.33 Hz, 2C), 127.49, 125.72, 124.93, 123.81, 115.82 (d, J = 21.42 Hz, 2C), 114.80, 113.33, 67.88, 61.07, 42.95, 40.62, 32.68, 29.05, 22.61 ppm. MS (m/z): 513 [M + Na]+.
N-{2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}-2-{4-[(6-hydroxyhexyl)oxy]-phenyl}acetamide (13n; ZHAWOC6643): The title compound 13n was obtained as a white solid in 26% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.20 (d, J = 1.86 Hz, 1H), 7.88 (dd, J = 8.19 Hz, 1.86 Hz, 1H), 7.83 (d, J = 8.19 Hz, 1H), 7.37–7.32 (m, 2H), 7.25–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.86 (m, 2H), 4.72 (s, 2H), 4.33 (t, J = 5.12 Hz, 1H), 3.92 (t, J = 6.51 Hz, 2H), 3.63 (s, 2H), 3.38 (td, J = 6.32 Hz, 5.12 Hz, 2H), 1.73–1.64 (m, 2H), 1.47–1.28 (m, 6H) ppm. 13C-NMR (DMSO-d6): δ = 170.95, 167.92, 167.74, 161.92 (d, J = 242.69 Hz, 1C), 158.05, 145.31, 133.56, 133.46 (d, J = 3.11 Hz, 1C), 130.65, 130.09 (d, J = 8.39 Hz, 2C), 127.50, 125.73, 124.94, 123.82, 115.83 (d, J = 21.32 Hz, 2C), 114.82, 113.34, 67.82, 61.11, 42.96, 40.63, 32.96, 29.22, 25.91, 25.75 ppm. MS (m/z): 505 [M + H]+.
N-{2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}-2-{4-[(7-hydroxyheptyl)oxy]-phenyl}acetamide (13o; ZHAWOC6636): The title compound 13o was obtained as a white solid in 53% yield: 1H-NMR (DMSO-d6): δ = 10.74 (s, 1H), 8.20 (d, J = 1.83 Hz, 1H), 7.89 (dd, J = 8.18 Hz, 1.83 Hz, 1H), 7.83 (d, J = 8.18 Hz, 1H), 7.37–7.32 (m, 2H), 7.25–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.86 (m, 2H), 4.72 (s, 2H), 4.32 (t, J = 5.11 Hz, 1H), 3.92 (t, J = 6.48 Hz, 2H), 3.63 (s, 2H), 3.37 (td, J = 6.35 Hz, 5.11 Hz, 2H), 1.73–1.64 (m, 2H), 1.45–1.23 (m, 8H) ppm. 13C-NMR (DMSO-d6): δ = 170.94, 167.90, 167.72, 161.90 (d, J = 243.10 Hz, 1C), 158.03, 145.31, 133.55, 133.45 (d, J = 3.10 Hz, 1C), 130.64, 130.07 (d, J = 8.23 Hz, 2C), 127.49, 125.71, 124.93, 123.81, 115.82 (d, J = 21.48 Hz, 2C), 114.80, 113.33, 67.83, 61.14, 42.96, 40.62, 32.94, 29.14, 29.13, 26.04, 25.93 ppm. MS (m/z): 519 [M + H]+.
N-{2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}-2-{4-[(8-hydroxyoctyl)oxy]-phenyl}acetamide (13p; ZHAWOC6639): The title compound 13p was obtained as a white solid in 24% yield: 1H-NMR (DMSO-d6): δ = 10.73 (s, 1H), 8.20 (d, J = 1.88 Hz, 1H), 7.89 (dd, J = 8.20 Hz, 1.88 Hz, 1H), 7.83 (d, J = 8.20 Hz, 1H), 7.37–7.32 (m, 2H), 7.25–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.86 (m, 2H), 4.72 (s, 2H), 4.31 (t, J = 5.12 Hz, 1H), 3.92 (t, J = 6.57 Hz, 2H), 3.63 (s, 2H), 3.37 (td, J = 6.48 Hz, 5.12 Hz, 2H), 1.73–1.64 (m, 2H), 1.45–1.21 (m, 10H) ppm. 13C-NMR (DMSO-d6): δ = 170.94, 167.90, 167.72, 161.90 (d, J = 243.48 Hz, 1C), 158.03, 145.30, 133.55, 133.44 (d, J = 3.05 Hz, 1C), 130.64, 130.07 (d, J = 8.38 Hz, 2C), 127.48, 125.72, 124.93, 123.81, 115.82 (d, J = 21.51 Hz, 2C), 114.80, 113.33, 67.84, 61.17, 42.95, 40.62, 32.99, 29.38, 29.30, 29.15, 25.97, 25.93 ppm. MS (m/z): 533 [M + H]+.
5-(4-{[(2-Methyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)pentanoic acid (14a; ZHAWOC6649): The alcohol (13a) (27 mg, 0.07 mmol), TEMPO (24 mg, 0.02 mmol) and 0.67 M aqueous sodium phosphate (0.80 ml) in acetonitrile (1.00 mL) were stirred and heated to 40 °C. NaClO2 (40 mg, in 0.12 mL water) and 0.25% NaOCl (0.05 mL) were added in parallel dropwise and it was stirred for 4 h. After cooling to ambient temperature, water (1.0 mL) was added and the mixture was poured in an ice cold solution of Na2SO3 (0.12 g in 2 mL water). The aqueous phase was extracted with diethyl ether (10 mL), acidified with 10% citric acid and again extracted with diethyl ether (2 × 10 mL). The organic phases were dried over sodium sulfate and concentrated in vacuum to obtain the title compound 14a as a white solid (10 mg, 36% yield, purity 96%): 1H-NMR (DMSO-d6): δ = 12.04 (br. s, 1H), 10.75 (s, 1H), 8.18 (d, J = 1.82 Hz, 1H), 7.86 (dd, J = 8.15 Hz, 1.82 Hz, 1H), 7.80 (d, J = 8.15 Hz, 1H), 7.26–7.22 (m, 2H), 6.90–6.86 (m, 2H), 3.93 (t, J = 6.25 Hz, 2H), 3.63 (s, 2H), 3.00 (s, 3H), 2.23 (t, J = 7.43 Hz, 2H), 1.74–1.67 (m, 2H), 1.66–1.59 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 170.90, 168.26, 168.13, 157.99, 145.06, 133.81, 130.63, 127.58, 126.07, 124.54, 123.53, 114.81, 113.11, 67.61, 42.96, 34.34, 28.70, 24.19, 21.90 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C22H22N2O6: 410.1478, found: 410.1473.
In analogy to ZHAWOC6649 the following derivatives were synthesized:
2-(4-{[(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)acetic acid (14b; ZHAWOC5474): The title compound 14b was obtained as a white solid in 38% yield and 98% purity: 1H-NMR (DMSO-d6): δ = 11.54 (s, 1H), 8.27 (d, J = 1.80 Hz, 1H), 7.99 (dd, J = 8.26 Hz, 1.80 Hz, 1H), 7.80 (d, J = 8.26 Hz, 1H), 7.34–7.23 (m, 5H), 7.18–7.13 (m, 2H), 6.77–6.72 (m, 2H), 4.73 (s, 2H), 4.13 (s, 2H), 3.62 (s, 2H) ppm. 13C-NMR (DMSO-d6): δ = 171.34, 171.20, 168.02, 167.83, 158.38, 145.76, 137.25, 133.43, 130.17, 129.04, 127.82, 127.79, 126.93, 125.44, 124.75, 123.98, 114.77, 113.46, 68.32, 42.95, 41.27 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C25H20N2O6: 444.1321, found: 444.1330.
3-(4-{[(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)propanoic acid (14c; ZHAWOC4765): The title compound 14c was obtained as a white solid in 86% yield and 98% purity: 1H-NMR (DMSO-d6): δ = 11.94 (br. s, 1H), 10.77 (s, 1H), 8.21 (d, J = 1.75 Hz, 1H), 7.90 (dd, J = 8.22 Hz, 1.75 Hz, 1H), 7.83 (d, J = 8.22 Hz, 1H), 7.35–7.22 (m, 7H), 6.91–6.87 (m, 2H), 4.73 (s, 2H), 4.14 (t, J = 6.02 Hz, 2H), 3.64 (s, 2H), 2.67 (t, J = 6.02 Hz, 2H) ppm. 13C-NMR (DMSO-d6): δ = 172.81, 171.71, 170.91, 167.94, 167.77, 157.70, 145.32, 137.21, 133.53, 130.69, 129.04, 127.85, 127.82, 125.71, 124.91, 123.82, 114.80, 113.34, 64.08, 42.93, 41.31, 34.67 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C26H22N2O6: 459.1557, found: 459.1538.
4-(4-{[(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)butanoic acid (14d; ZHAWOC4766): The title compound 14d was obtained as a white solid in 67% yield and 95% purity: 1H-NMR (DMSO-d6): δ = 12.14 (br. s, 1H), 10.73 (s, 1H), 8.21 (d, J = 1.80 Hz, 1H), 7.89 (dd, J = 8.21 Hz, 1.80 Hz, 1H), 7.84 (d, J = 8.21 Hz, 1H), 7.34–7.21 (m, 7H), 6.90–6.86 (m, 2H), 4.73 (s, 2H), 3.95 (t, J = 6.41 Hz, 2H), 3.64 (s, 2H), 2.36 (t, J = 6.41 Hz, 2H), 1.92 (quint., J = 6.75 Hz, 2H) ppm. 13C-NMR (DMSO-d6): δ = 174.55, 170.92, 167.94, 167.77, 157.86, 145.30, 137.21, 133.54, 130.66, 129.05, 127.86, 127.83, 127.66, 125.72, 124.93, 123.82, 114.84, 113.33, 67.02, 42.94, 41.31, 30.61, 24.72 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C27H24N2O6: 473.1713, found: 473.1699.
5-(4-{[(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)pentanoic acid (14e; ZHAWOC5131): The title compound 14e was obtained as a white solid in 62% yield and 99% purity: 1H-NMR (DMSO-d6): δ = 10.75 (s, 1H), 8.21 (d, J = 1.83 Hz, 1H), 7.89 (dd, J = 8.25 Hz, 1.83 Hz, 1H), 7.83 (d, J = 8.25 Hz, 1H), 7.34–7.21 (m, 7H), 6.90–6.86 (m, 2H), 4.73 (s, 2H), 3.93 (t, J = 6.18 Hz, 2H), 3.63 (s, 2H), 2.27 (t, J = 7.35 Hz, 2H), 1.74–1.67 (m, 2H), 1.66–1.59 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 174.85, 170.93, 167.94, 167.76, 157.97, 145.31, 137.21, 133.53, 130.64, 129.04, 127.85, 127.82, 127.55, 125.71, 124.91, 123.81, 114.81, 113.33, 67.55, 42.95, 41.31, 33.81, 28.60, 21.71 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C28H26N2O6: 487.1870, found: 487.1853.
6-(4-{[(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)hexanoic acid (14f; ZHAWOC5133): The title compound 14f was obtained as a white solid in 96% yield and 94% purity: 1H-NMR (DMSO-d6): δ = 12.01 (br. s, 1H), 10.76 (s, 1H), 8.22 (d, J = 1.80 Hz, 1H), 7.90 (dd, J = 8.27 Hz, 1.80 Hz, 1H), 7.84 (d, J = 8.27 Hz, 1H), 7.36–7.21 (m, 7H), 6.90–6.86 (m, 2H), 4.74 (s, 2H), 3.93 (t, J = 6.48 Hz, 2H), 3.64 (s, 2H), 2.20 (t, J = 7.26 Hz, 2H), 1.73–1.66 (m, 2H), 1.59–1.51 (m, 2H); 1.44–1.37 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 177.22, 171.23, 168.02, 167.83, 158.00, 145.78, 137.25, 133.44, 130.63, 129.04, 127.83, 127.79, 127.77, 125.45, 124.77, 123.96, 114.67, 113.47, 67.99, 42.86, 41.27, 38.78, 29.35, 26.76, 26.37 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C29H28N2O6: 501.2026, found: 501.2027.
7-(4-{[(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)heptanoic acid (14g; ZHAWOC6650): The title compound 14g was obtained as a white solid in 55% yield and 84% purity: 1H-NMR (DMSO-d6): δ = 11.72 (br. s, 1H), 10.73 (s, 1H), 8.21 (d, J = 1.81 Hz, 1H), 7.89 (dd, J = 8.27 Hz, 1.81 Hz, 1H), 7.84 (d, J = 8.27 Hz, 1H), 7.40–7.20 (m, 7H), 6.90–6.85 (m, 2H), 4.74 (s, 2H), 3.92 (t, J = 6.41 Hz, 2H), 3.63 (s, 2H), 2.20 (t, J = 7.28 Hz, 2H), 1.73–1.64 (m, 2H), 1.55–1.47 (m, 2H); 1.44–1.36 (m, 2H), 1.35–1.28 (m, 2H) ppm. 13C-NMR ( DMSO-d6): δ = 174.95, 170.96, 167.96, 167.79, 158.04, 145.32, 137.23, 133.56, 130.65, 129.06, 127.88, 127.84, 127.65, 125.74, 124.95, 123.83, 114.82, 113.34, 67.78, 42.96, 41.32, 34.06, 29.01, 28.75, 25.72, 24.90 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C30H30N2O6: 514.2104, found: 514.2096.
8-(4-{[(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)octanoic acid (14h; ZHAWOC6651): The title compound 14h was obtained as a white solid in 50% yield and 92% purity: 1H-NMR (DMSO-d6): δ = 10.79 (s, 1H), 8.21 (d, J = 1.81 Hz, 1H), 7.89 (dd, J = 8.21 Hz, 1.81 Hz, 1H), 7.84 (d, J = 8.21 Hz, 1H), 7.35–7.21 (m, 7H), 6.89–6.85 (m, 2H), 4.73 (s, 2H), 3.92 (t, J = 6.48 Hz, 2H), 3.63 (s, 2H), 2.16 (t, J = 7.31 Hz, 2H), 1.71–1.64 (m, 2H), 1.52–1.45 (m, 2H); 1.42–1.22 (m, 6H) ppm. 13C-NMR (DMSO-d6): δ = 170.97, 167.96, 167.79, 158.04, 145.35, 137.23, 133.55, 130.65, 129.06, 127.87, 127.84, 127.52, 125.71, 124.93, 123.83, 114.82, 113.35, 67.82, 42.95, 41.31, 34.53, 29.10, 29.04, 28.97, 25.87, 25.08 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C31H32N2O6: 528.2260, found: 528.2245.
9-(4-{[(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)nonanoic acid (14i; ZHAWOC6941): The title compound 14i was obtained as a white solid in 39% yield and 96% purity: 1H-NMR (DMSO-d6): δ = 11.99 (br. s, 1H), 10.74 (s, 1H), 8.21 (d, J = 1.80 Hz, 1H), 7.89 (dd, J = 8.21 Hz, 1.80 Hz, 1H), 7.84 (d, J = 8.21 Hz, 1H), 7.35–7.21 (m, 7H), 6.89–6.85 (m, 2H), 4.73 (s, 2H), 3.92 (t, J = 6.47 Hz, 2H), 3.63 (s, 2H), 2.18 (t, J = 7.34 Hz, 2H), 1.72–1.64 (m, 2H), 1.52–1.45 (m, 2H); 1.42–1.35 (m, 2H), 1.33–1.22 (m, 6H) ppm. 13C-NMR (DMSO-d6): δ = 174.99, 170.94, 167.94, 167.77, 158.03, 145.31, 137.21, 133.54, 130.63, 129.04, 127.86, 127.83, 127.49, 125.71, 124.93, 123.81, 114.81, 113.33, 67.84, 42.96, 41.31, 34.20, 29.17, 29.13, 29.11, 28.98, 25.95, 24.98 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C32H34N2O6: 542.2417, found: 542.2406.
10-(4-{[(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)decanoic acid (14j; ZHAWOC6942): The title compound 14j was obtained as a white solid in 33% yield and 98% purity: 1H-NMR (DMSO-d6): δ = 12.01 (br. s, 1H), 10.76 (s, 1H), 8.21 (d, J = 1.86 Hz, 1H), 7.89 (dd, J = 8.15 Hz, 1.86 Hz, 1H), 7.84 (d, J = 8.15 Hz, 1H), 7.35–7.21 (m, 7H), 6.89–6.85 (m, 2H), 4.73 (s, 2H), 3.92 (t, J = 6.51 Hz, 2H), 3.63 (s, 2H), 2.17 (t, J = 7.51 Hz, 2H), 1.72–1.64 (m, 2H), 1.52–1.44 (m, 2H); 1.43–1.35 (m, 2H), 1.33–1.20 (m, 8H) ppm. 13C-NMR (DMSO-d6): δ = 170.95, 167.94, 167.77, 158.02, 145.32, 137.21, 133.54, 130.63, 129.04, 127.86, 127.82, 127.50, 125.71, 124.92, 123.81, 114.81, 113.33, 67.83, 42.96, 41.31, 34.29, 29.33, 29.18, 29.16, 29.13, 29.02, 25.96, 25.03 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C33H36N2O6: 556.2573, found: 556.2568.
3-{4-[({2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}carbamoyl)methyl]-phenoxy}propanoic acid (14k; ZHAWOC6644): The title compound 14k was obtained as a white solid in 62% yield and 97% purity: 1H-NMR (DMSO-d6): δ = 12.35 (br. s, 1H), 10.74 (s, 1H), 8.21 (d, J = 1.86 Hz, 1H), 7.89 (dd, J = 8.24 Hz, 1.86 Hz, 1H), 7.83 (d, J = 8.24 Hz, 1H), 7.37–7.31 (m, 2H), 7.26–7.22 (m, 2H), 7.17–7.12 (m, 2H), 6.91–6.86 (m, 2H), 4.72 (s, 2H), 4.14 (t, J = 6.10 Hz, 2H), 3.64 (s, 2H), 2.66 (t, J = 6.10 Hz, 2H) ppm. 13C-NMR (DMSO-d6): δ = 172.71, 170.90, 167.91, 167.73, 161.91 (d, J = 239.42 Hz, 1C), 157.71, 145.30, 133.55, 133.45 (d, J = 3.29 Hz, 1C), 130.69, 130.07 (d, J = 8.27 Hz, 2C), 127.81, 125.73, 124.93, 123.82, 115.82 (d, J = 21.45 Hz, 2C), 114.81, 113.34, 64.09, 42.93, 40.63, 34.66 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C26H21FN2O6: 476.1384, found: 476.1391.
4-{4-[({2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}carbamoyl)methyl]-phenoxy}butanoic acid (14l; ZHAWOC5463): The title compound 14l was obtained as a white solid in 97% yield and 97% purity: 1H-NMR (DMSO-d6): δ = 11.99 (br. s, 1H), 10.74 (s, 1H), 8.21 (d, J = 1.80 Hz, 1H), 7.89 (dd, J = 8.34 Hz, 1.80 Hz, 1H), 7.84 (d, J = 8.34 Hz, 1H), 7.37–7.32 (m, 2H), 7.26–7.22 (m, 2H), 7.17–7.12 (m, 2H), 6.91–6.86 (m, 2H), 4.72 (s, 2H), 3.96 (t, J = 6.39 Hz, 2H), 3.64 (s, 2H), 2.73 (t, J = 7.37 Hz, 2H), 1.92 (quint., J = 6.69 Hz, 2H) ppm. 13C-NMR (DMSO-d6): δ = 174.54, 170.91, 167.90, 167.72, 161.91 (d, J = 243.34 Hz, 1C), 157.85, 145.30, 133.54, 133.44 (d, J = 3.06 Hz, 1C), 130.66, 130.07 (d, J = 8.32 Hz, 2C), 127.66, 125.71, 124.92, 123.81, 115.81 (d, J = 21.45 Hz, 2C), 114.83, 113.33, 67.00, 42.94, 40.62, 30.57, 24.70 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C27H23FN2O6: 490.1540, found: 490.1538.
5-{4-[({2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}carbamoyl)methyl]-phenoxy}pentanoic acid (14m; ZHAWOC5684): The title compound 14m was obtained as a white solid in 50% yield and 96% purity: 1H-NMR (DMSO-d6): δ = 11.92 (br. s, 1H), 10.74 (s, 1H), 8.20 (d, J = 1.82 Hz, 1H), 7.89 (dd, J = 8.25 Hz, 1.82 Hz, 1H), 7.83 (d, J = 8.25 Hz, 1H), 7.37–7.31 (m, 2H), 7.26–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.86 (m, 2H), 4.72 (s, 2H), 3.94 (t, J = 6.16 Hz, 2H), 3.63 (s, 2H), 2.27 (t, J = 6.41 Hz, 2H), 1.75–1.67 (m, 2H), 1.66–1.59 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 174.83, 170.93, 167.90, 167.72, 161.90 (d, J = 243.25 Hz, 1C), 157.97, 145.30, 133.54, 133.44 (d, J = 3.03 Hz, 1C), 130.64, 130.07 (d, J = 8.24 Hz, 2C), 127.55, 125.71, 124.93, 123.81, 115.81 (d, J = 21.45 Hz, 2C), 114.81, 113.33, 67.54, 42.95, 40.63, 33.76, 28.59, 21.69 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C28H25FN2O6: 505.1776, found: 505.1758.
6-{4-[({2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}carbamoyl)methyl]-phenoxy}hexanoic acid (14n; ZHAWOC6645): The title compound 14n was obtained as a white solid in 59% yield and 97% purity: 1H-NMR (DMSO-d6): δ = 11.98 (br. s, 1H), 10.73 (s, 1H), 8.20 (d, J = 1.82 Hz, 1H), 7.89 (dd, J = 8.23 Hz, 1.82 Hz, 1H), 7.83 (d, J = 8.23 Hz, 1H), 7.37–7.31 (m, 2H), 7.26–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.86 (m, 2H), 4.72 (s, 2H), 3.92 (t, J = 6.45 Hz, 2H), 3.63 (s, 2H), 2.22 (t, J = 7.29 Hz, 2H), 1.73–1.65 (m, 2H), 1.59–1.51 (m, 2H), 1.45–1.37 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 174.88, 170.93, 167.90, 167.73, 161.90 (d, J = 243.07 Hz, 1C), 158.01, 145.30, 133.55, 133.45 (d, J = 3.11 Hz, 1C), 130.64, 130.07 (d, J = 8.24 Hz, 2C), 127.52, 125.72, 124.93, 123.81, 115.82 (d, J = 21.46 Hz, 2C), 114.81, 113.33, 67.75, 42.95, 40.63, 34.09, 28.91, 25.63, 24.74 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C29H27FN2O6: 518.1853, found: 518.1840.
7-{4-[({2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}carbamoyl)methyl]-phenoxy}heptanoic acid (14o; ZHAWOC6637): The title compound 14o was obtained as a white solid in 56% yield and 99% purity: 1H-NMR (DMSO-d6): δ = 12.03 (br. s, 1H), 10.74 (s, 1H), 8.20 (d, J = 1.85 Hz, 1H), 7.89 (dd, J = 8.24 Hz, 1.85 Hz, 1H), 7.83 (d, J = 8.24 Hz, 1H), 7.37–7.31 (m, 2H), 7.26–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.86 (m, 2H), 4.72 (s, 2H), 3.92 (t, J = 6.47 Hz, 2H), 3.63 (s, 2H), 2.19 (t, J = 7.51 Hz, 2H), 1.72–1.64 (m, 2H), 1.54–1.47 (m, 2H), 1.43–1.36 (m, 2H), 1.35–1.28 (m, 2H) ppm. 13C-NMR (DMSO-d6): δ = 174.98, 170.94, 167.91, 167.73, 161.90 (d, J = 243.56 Hz, 1C), 158.03, 145.31, 133.55, 133.46 (d, J = 2.14 Hz, 1C), 130.64, 130.08 (d, J = 8.27 Hz, 2C), 127.51, 125.72, 124.93, 123.81, 115.82 (d, J = 21.52 Hz, 2C), 114.81, 113.34, 67.78, 42.95, 40.63, 34.20, 29.02, 28.78, 25.73, 24.96 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C30H29FN2O6: 532.2010, found: 532.2013.
8-{4-[({2-[(4-Fluorophenyl)methyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}carbamoyl)methyl]-phenoxy}octanoic acid (14p; ZHAWOC6646): The title compound 14p was obtained as a white solid in 30% yield and 99% purity: 1H-NMR (DMSO-d6): δ = 12.00 (br. s, 1H), 10.74 (s, 1H), 8.20 (d, J = 1.87 Hz, 1H), 7.89 (dd, J = 8.27 Hz, 1.87 Hz, 1H), 7.83 (d, J = 8.27 Hz, 1H), 7.37–7.31 (m, 2H), 7.26–7.21 (m, 2H), 7.17–7.11 (m, 2H), 6.90–6.86 (m, 2H), 4.72 (s, 2H), 3.92 (t, J = 6.47 Hz, 2H), 3.63 (s, 2H), 2.19 (t, J = 7.39 Hz, 2H), 1.71–1.64 (m, 2H), 1.53–1.45 (m, 2H), 1.42–1.35 (m, 2H), 1.34–1.24 (m, 4H) ppm. 13C-NMR (DMSO-d6): δ = 174.98, 170.94, 167.90, 167.73, 161.90 (d, J = 242.92 Hz, 1C), 158.03, 145.31, 133.55, 133.45 (d, J = 3.11 Hz, 1C), 130.63, 130.07 (d, J = 8.32 Hz, 2C), 127.49, 125.71, 124.93, 123.81, 115.82 (d, J = 21.55 Hz, 2C), 114.81, 113.33, 67.82, 42.95, 40.62, 34.20, 29.09, 28.98, 28.94, 25.86, 24.94 ppm. HRMS-TOF (m/z): [M + H]+ calculated for C31H31FN2O6: 546.2166, found: 546.2169.